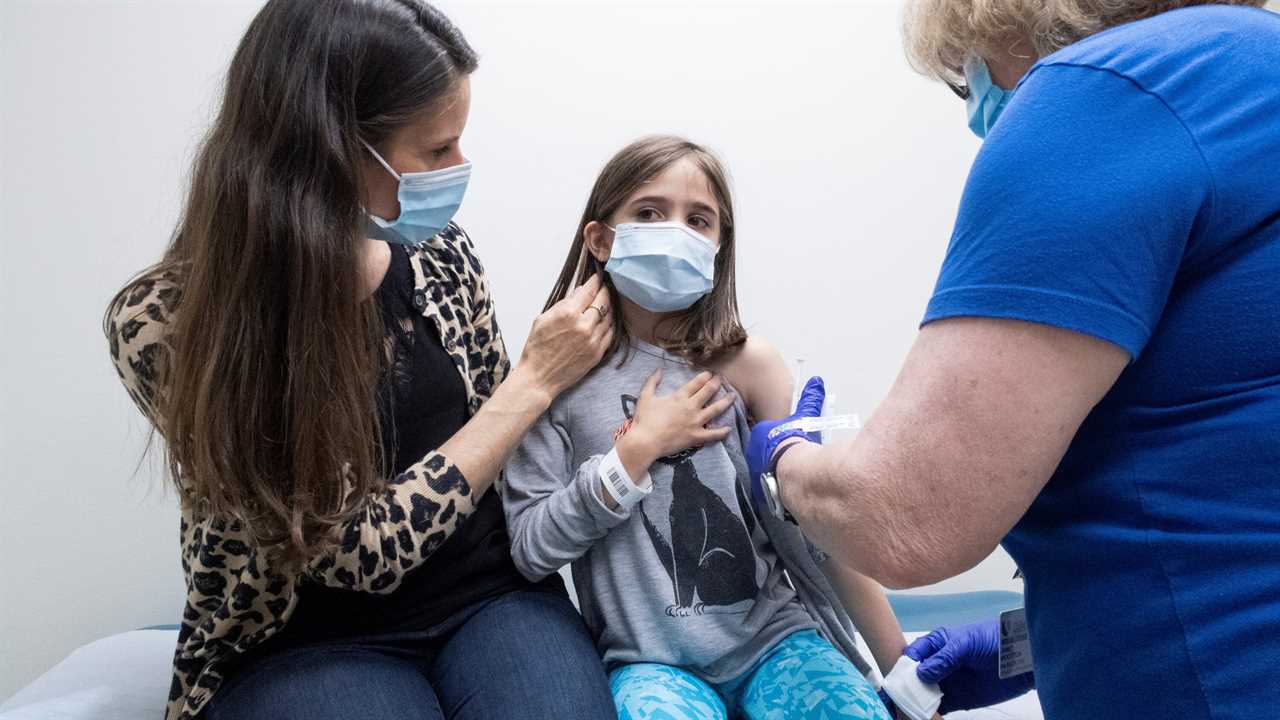
WASHINGTON — At the urging of federal regulators, two coronavirus vaccine makers are expanding the size of their studies in children ages 5 to 11 — a precautionary measure intended to detect rare side effects including heart inflammation problems that turned up in vaccinated people younger than 30.
Appearing at a televised town-hall-style meeting in Ohio last week, President Biden said that emergency clearance for pediatric vaccines would come “soon.” The White House has declined to be more specific on the timeline, and it was unclear how much of an impact, if any, expanding the studies would have on when vaccines could be authorized for young children.
Multiple people familiar with the trials said the Food and Drug Administration had indicated to the two vaccine makers, Pfizer-BioNTech and Moderna, that the size and the scope of their pediatric studies, as initially envisioned, were inadequate to detect the rare side effects. Those include myocarditis, an inflammation of the heart muscle, and pericarditis, inflammation of the lining around the heart.
Questions about vaccinating children — including those under 12 — are of huge interest to parents and teachers. Regulators will be required to balance potential side effects of coronavirus vaccination against the risks of Covid-19.
Members of a Centers for Disease Control and Prevention advisory committee have said that the benefits of shots for people older than 12 greatly outweigh the risks, including of heart problems. Children 12 and older have been eligible for the Pfizer shot for months even as studies continue on the safety of the Pfizer and Moderna vaccines for younger children.
The F.D.A. has asked the companies to include 3,000 children in the 5-to-11-year-old age group, the group of younger children for whom results were expected first, according to people familiar with the situation. That is double the original number of study participants envisioned in the Pfizer trial for that age group, two people said. The study initially sought to enroll 4,500 patients under 12, split into a group of 5- to 11-year-olds and two younger age groups, according to a government website.
A spokesman for Moderna, Ray Jordan, confirmed that the company intended to expand its trial “to enroll a larger safety database, which increases the likelihood of detecting rarer events,” and expected to seek emergency authorization in “winter 2021/early 2022.”
The Moderna trial began recruiting patients in March with the aim of enrolling 6,795 participants under 12, also split equally into three age groups, including a group of 6- to 11- year-olds. Mr. Jordan said the company was “actively discussing” a proposal with the F.D.A. to expand the study.
Pfizer is on a faster timetable than Moderna, and may be able to meet the F.D.A.’s expectations on a bigger trial size and still file a request to expand emergency authorization of its vaccine by the end of September. Reviewing all the safety and efficacy data will most likely take regulators at least a few weeks.
Pfizer has previously said it expects to have results for the 5-to-11-year-old group in September, with results for children ages 2 to 5 shortly after. Results for the youngest children, between 6 months and 2 years old, are expected in October or November. A spokeswoman said on Monday that the company had no updates on its timetable.
A spokeswoman for the F.D.A., Stephanie Caccomo, declined to offer specifics. “While we cannot comment on individual interactions with sponsors, we do generally work with sponsors to ensure the number of participants in clinical trials are of adequate size to detect safety signals,” she said in an email.
In June, the C.D.C. published data showing that the two vaccines may have caused myocarditis and pericarditis in more than 1,200 Americans, including about 500 who were younger than 30. The symptoms typically appeared within two weeks and were more common in young men and boys. The rate was low: fewer than 13 cases per one million second doses administered.
Most cases were mild and quickly cleared up, the researchers said. And Dr. Paul A. Offit, an infectious disease specialist who previously served on the C.D.C.’s Advisory Committee on Immunization Practices, which makes recommendations on vaccine use in the United States, noted that infection with the coronavirus also carried a risk.
If expanding the trials were to cause a delay in authorizing vaccines for pediatric use, he said, that would also put children at risk. “There’s always a human price to pay for knowledge,” he said. Of the heart ailments, he said: “It’s rare. It’s generally short lived and self resolving. It’s also a consequence of natural infection.”






