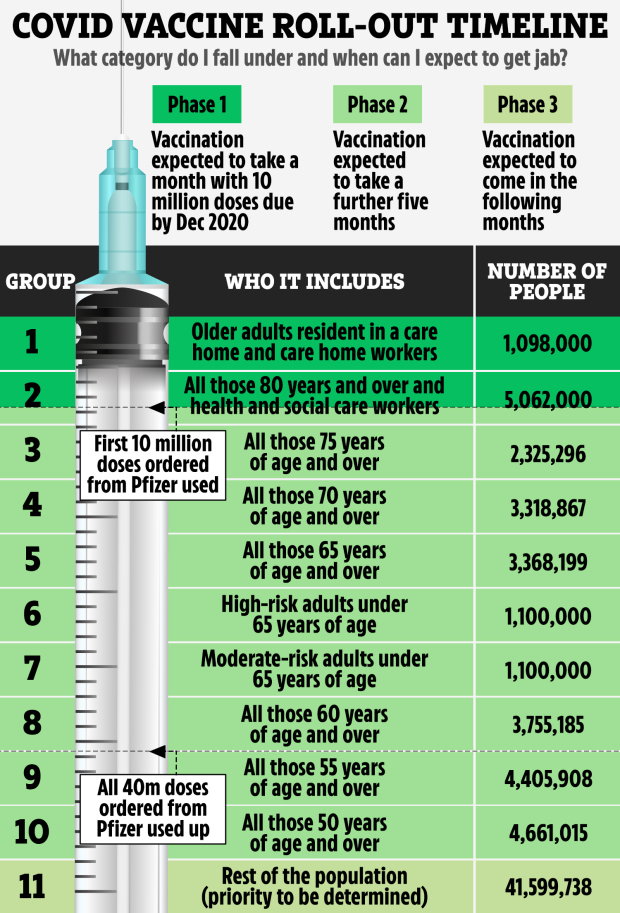
HUNDREDS of clinical trials for a Covid-19 vaccine have been in the works in labs across the world with one finally giving hope for a cure.
The messenger RNA (mRNA) vaccine developed by pharmaceutical giant Pfizer is said to be now 95 per cent effective, as senior advisers hope to roll out the vaccine before Christmas.
What coronavirus vaccines are available?
Pfizer and BioNTech
Pfizer and BioNTech co-developed a jab known as a messenger RNA (mRNA) vaccine.
Conventional vaccines are produced using weakened forms of the virus, but mRNAs use only the virus’s genetic code.
An mRNA vaccine is injected into the body where it enters cells and tells them to create antigens.
These antigens are recognised by the immune system and prepare it to fight coronavirus.
No actual virus is needed to create an mRNA vaccine.
This means the rate at which the vaccine can be produced is dramatically accelerated.
As a result, mRNA vaccines have been hailed as potentially offering a rapid solution to new outbreaks of infectious diseases.
They can also be modified reasonably quickly if, for example, a virus develops mutations and begins to change.
mRNA vaccines are also cheaper to produce than traditional vaccines. But both will play an important role in tackling Covid-19.
Dr Onyema Ogbuagu, who is serving as principal investigator for Pfizer’s vaccine clinical trials, said there’s no way to know for sure when its vaccine will be available, but researchers hope the trial results will be in by November.
Dr Ogbuagu said: “So I think that the most optimistic expectations around the vaccine availability would be the first quarter of 2021.”
Britain has secured 40 million doses the Pfizer-BioNTech coronavirus vaccine – enough to immunise a third of the UK population.
GlaxoSmithKleine and Sanofi
Another vaccine is being developed by GSK and Sanofi and is based on the DNA of the virus.
It takes recombinant protein-based technology used to produce the seasonal flu vaccine, which is then combined with GSK’s established pandemic adjuvant technology.
Sanofi has said that regulatory approval could be achieved by the first half of 2021 if trials are successful.
Human clinical studies of the vaccine began in September followed by a Phase 3 study in December 2020.
In the meantime, Sanofi and GSK are scaling up manufacturing to produce up to one billion doses a year overall.
AstraZeneca and Oxford University
Another of the vaccine frontrunners includes the treatment developed by Oxford University in conjunction with AstraZeneca, with millions of doses already stockpiled.
But with patients needing two jabs, 28 days apart, inoculating vast numbers of Brits is set to be a logistical nightmare.
The Oxford/AstraZeneca vaccine is made from virus ChAdOx1, a weakened version of a common cold virus (adenovirus) that causes infections in chimpanzees.
The vaccine is still in testing, but a major effort has been ordered to have the world-leading hospital in London ready to go as soon as it is given the green light.
The UK government has ordered 100 million doses of the AstraZeneca vaccine.
Moderna
The jab uses the same RNA technology as the Pfizer vaccine and has been found to be 94 per cent effective.
Trials on more than 30,000 people found that only five given the Moderna jab developed Covid – none with severe symptoms.
In comparison, 90 people given a dummy vaccine fell ill, according to the US biotechnology company.
However, the UK has no pre-orders of the jab but officials said they are in advanced talks to buy up millions.
But it will be spring 2021 “at the earliest” before doses are available to Brits – as the US firm is scaling up its European supply chain, a Government spokesman said.
The Moderna jab can be stored in a fridge for a month – meaning it would be much easier to distribute than the Pfizer frontrunner.
Scientists claim it remains stable at -20C for up to half a year, at refrigerated conditions for up to 30 days and at room temperature for up to 12 hours.
Juan Andres, Chief Technical Operations and Quality Officer at Moderna, said: “The ability to store our vaccine for up to 6 months at -20C including up to 30 days at normal refrigerator conditions after thawing is an important development and would enable simpler distribution.”
Moderna also included many high-risk and elderly people in its major clinical trial, which experts claim makes the results more relevant to those most vulnerable to Covid.
How will the vaccine work?
The proposed vaccines all work in slightly different ways.
Pfizer’s vaccine mRNA vaccine would be administered into the body via two jabs about three weeks apart.
The vaccine would enter the body’s cells and tell them to create antigens, then recognised by the immune system to fight coronavirus.
The GlaxoSmithKleine and Sanofi vaccine is based on the DNA of the virus and takes recombinant protein-based technology used to produce the seasonal flu vaccine, which is then combined with GSK’s established pandemic adjuvant technology.
Finally, the Oxford/AstraZeneca vaccine is made from virus ChAdOx1, a weakened version of a common cold virus (adenovirus) that causes infections in chimpanzees.
The first mass vaccination centres are planned for sites in major cities including Leeds, Hull and London.
The giant sites – manned by trainee nurses, physios and paramedics – will be able to treat tens of thousands of people daily.
They will be supported by hundreds of mobile vaccination units dotted nationwide, while roving teams will visit care homes and vulnerable Brits
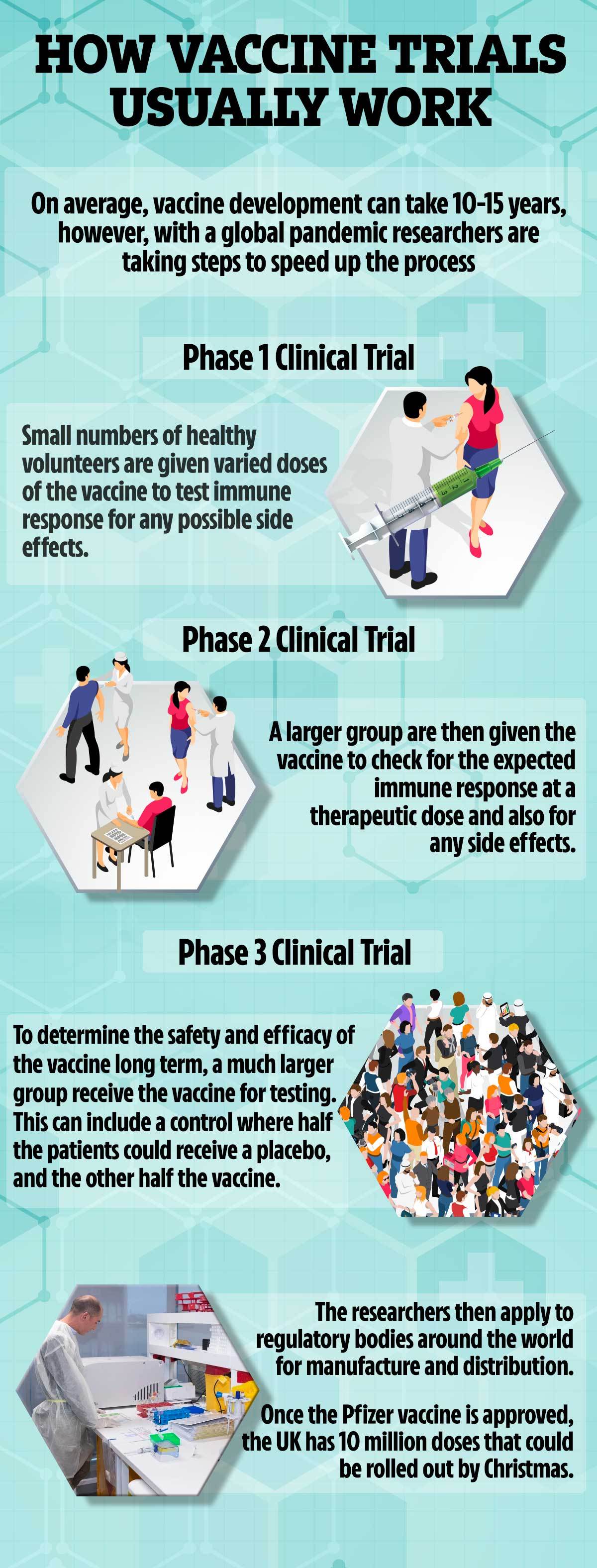
Are there any side effects to a Covid vaccine?
All vaccines undergo rigorous testing and have oversight from experienced regulators.
Some believe mRNA vaccines are safer for the patient as they do not rely on any element of the virus being injected into the body.
mRNA vaccines have been tried and tested in the lab and on animals but the coronavirus vaccine will be the first one licensed for use in humans.
The human trials of mRNA vaccines – involving tens of thousands of people – have been going on since early 2020 to show whether it is safe and effective.
Pfizer’s vaccine has been tested on 43,500 people in six countries and no safety concerns have been raised.
The first volunteers in the US said side effects were similar to a hangover.
Pfizer said it will continue to collect safety and long-term outcomes data from participants for two years.
A Health department spokesperson said: “A Covid-19 vaccine will only be deployed once it has been proven to be safe and effective through robust clinical trials and approved for use by the independent regulator.”