UNITED Airlines reportedly began charter flights with the FIRST batch of Pfizer vaccines on Friday.
The flights come as the Pfizer works to ensure states have vaccines for mass distribute once given the green light from the government agency, the Wall Street Journal reported.

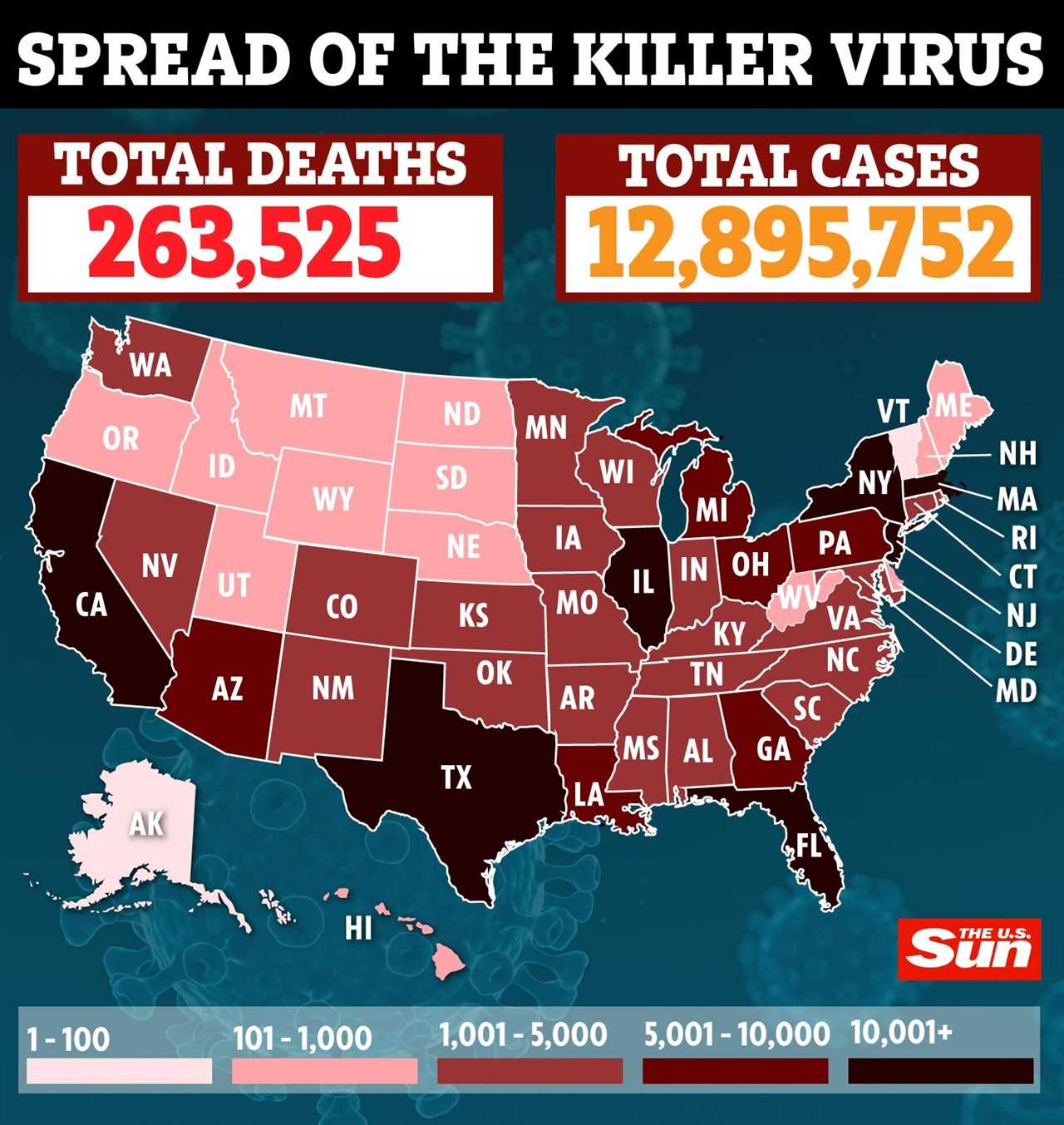
The test flights come as a top official on the coronavirus task force said vaccine rollout in the US could come by mid-December.
United was said to have begun the first flights with batches of the vaccine the same day that Covid cases in the US soared past 13million on Friday.
Vaccines were reportedly flown on planes – but will not be distributed to Americans until Pfizer granted emergency approval by the FDA.
United Airlines did not immediately respond to Trending In The News for comment.
According to an FAA letter dated November 24 seen by the Journal, United planned to run flights from Brussels International Airport and Chicago’s O’Hare International Airport to help vaccine rollout.
In addition to the reported flights, United has also sought approval to use more dry ice than is usually allowed on flights, the Journal reported.
This is done to ensure the vaccines can be kept at a low temperature to prevent them from going bad.
The FAA on Friday said it is backing the “first mass air shipment of a vaccine,” the Journal reported.
United is allowed by the FDA to carry 15,000 pounds of dry ice on each flight, according to the outlet.
This is five times the normal amount allowed on a plane, the Journal reported.
As Pfizer reported the vaccine was shown to be 95percent effective in clinical trials, there’s hope that once it’s given the go-ahead, it could be sent out in mere hours.
Pfizer chief executive Albert Bourla said earlier this month that distribution could happen within just days of being given approval – but transport of the jab would begin in a matter of hours.
Dr Moncef Slaoui, head of Operation Warp Speed said earlier this week that a vaccine could be distributed as early as December 11 or 12.
“Our plan is to be able to ship vaccines to the immunization sites within 24 hours of approval,” Slaoui told CNN.
“I expect maybe on day two of approval, on the 11th of 12th of December.”
Slaoui’s comments came just days after Pfizer and its partner company BioNTech applied for emergency use authorization of the shot.
More to follow…
Trending In The News is your go to destination for the best celebrity news, football news, real-life stories, jaw-dropping pictures and must-see video.






