PFIZER’S Covid vaccine could get the green light from British regulators by the end of this week.
A formal appraisal is about to start on the jab developed by US pharmaceutical giant Pfizer and German firm BioNTech, and doctors have been told to be ready on December 1.

Government sources told The Daily Telegraph that in a “best case scenario” a decision could be made in less than a week and the NHS could prepare to start giving out the jabs by December 1.
That would mean the vaccine gets UK approval before the US.
In the US, the Food and Drug Administration’s key meeting will not be held until December 10.
The head of the US vaccine programme, Dr Moncef Slaoui, told CNN the vaccine could be rolled out “maybe a day or two after approval, on the 11th or the 12th of December”.
In the UK, the Medicines and Healthcare products Regulatory Agency, an independent body, is responsible for the regulatory process and in charge of the timescale.
It was formally asked by Health Secretary Matt Hancock to check the Pfizer vaccine and approve it.
It has already been formally asked to assess the vaccine and will receive all the safety and efficacy data on either Monday or Tuesday.
The NHS has already drawn up plans to start administering vaccines from December 1.
It is hoped every adult in England will be vaccinated against Covid-19 by April next year.
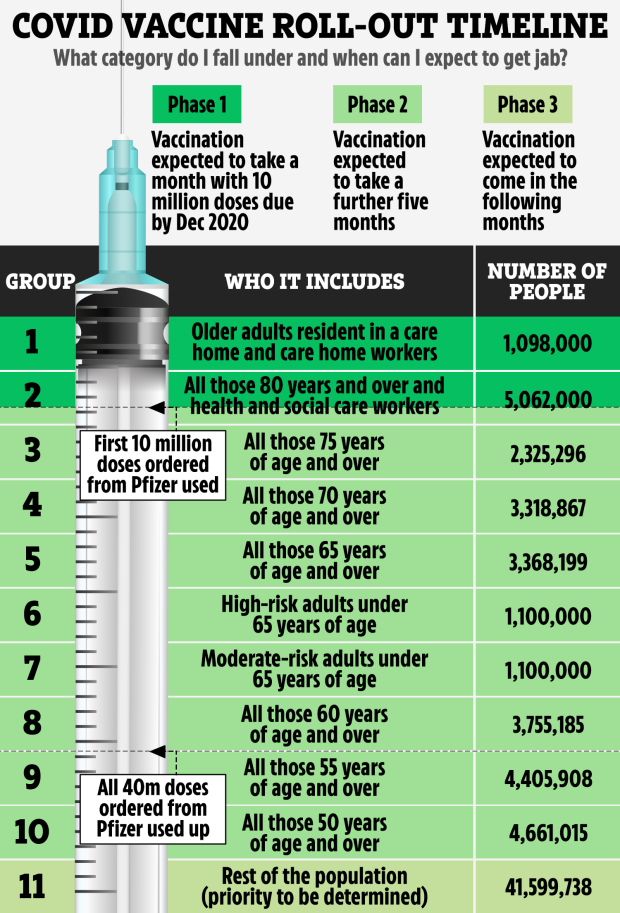
According to the timetable care home residents and staff, NHS workers and the elderly will be first in line with a further roll-out starting in the new year.
That schedule though depends on authorisation and the arrival of millions of vaccines.
The original draft timetable which was drawn up a week ago has already been amended following updated information from manufacturers.
Hancock said on Friday he was becoming “more and more confident” that life would be closer to normal in the spring.
Deputy chief medical officer Professor Jonathan Van-Tam has said the NHS intended to “move with as much pace as we can possibly muster” with just a few weeks’ difference between priority groups.
Up to 30,000 volunteers are being recruited to administer the Pfizer jabs, which have been found to be 95 per cent effective.
Around 28million doses of the vaccine will be delivered from 40-50 “large scale mass vaccination centres” which will be set up around the country in conference centres, stadiums and other sites.
Another 34million doses will be administered from 1,000 mass vaccination sites run by GPs, according to NHS plans.
The vaccine dealt with by GPs is likely to be the one developed by Oxford University and AstraZeneca, which can be stored more easily.
Both vaccines require two doses, given 28 days apart.
Pfizer has said it will deliver 10million of the 40million doses ordered by the UK by the end of the year.
Results from the trials of the Oxford/AstraZeneca vaccine are expected shortly.
So far Phase II trials have shown to be extremely promising, especially on the elderly.
Phase III trials are still ongoing but expected to be completed within weeks.
The government has ordered 100million doses of the AstraZeneca vaccine.