MODERNA’S “highly effective” coronavirus shot is linked to no serious side effects and could be the second Covid vaccine released across the US within days.
The biotechnology company’s vaccine has been given the green light to continue on its path to the public one day after the first batch of Food and Drug Administration-authorized (FDA) COVID-19 vaccine – made by Pfizer and BioNTech – arrived at hundreds of hospitals across the country.
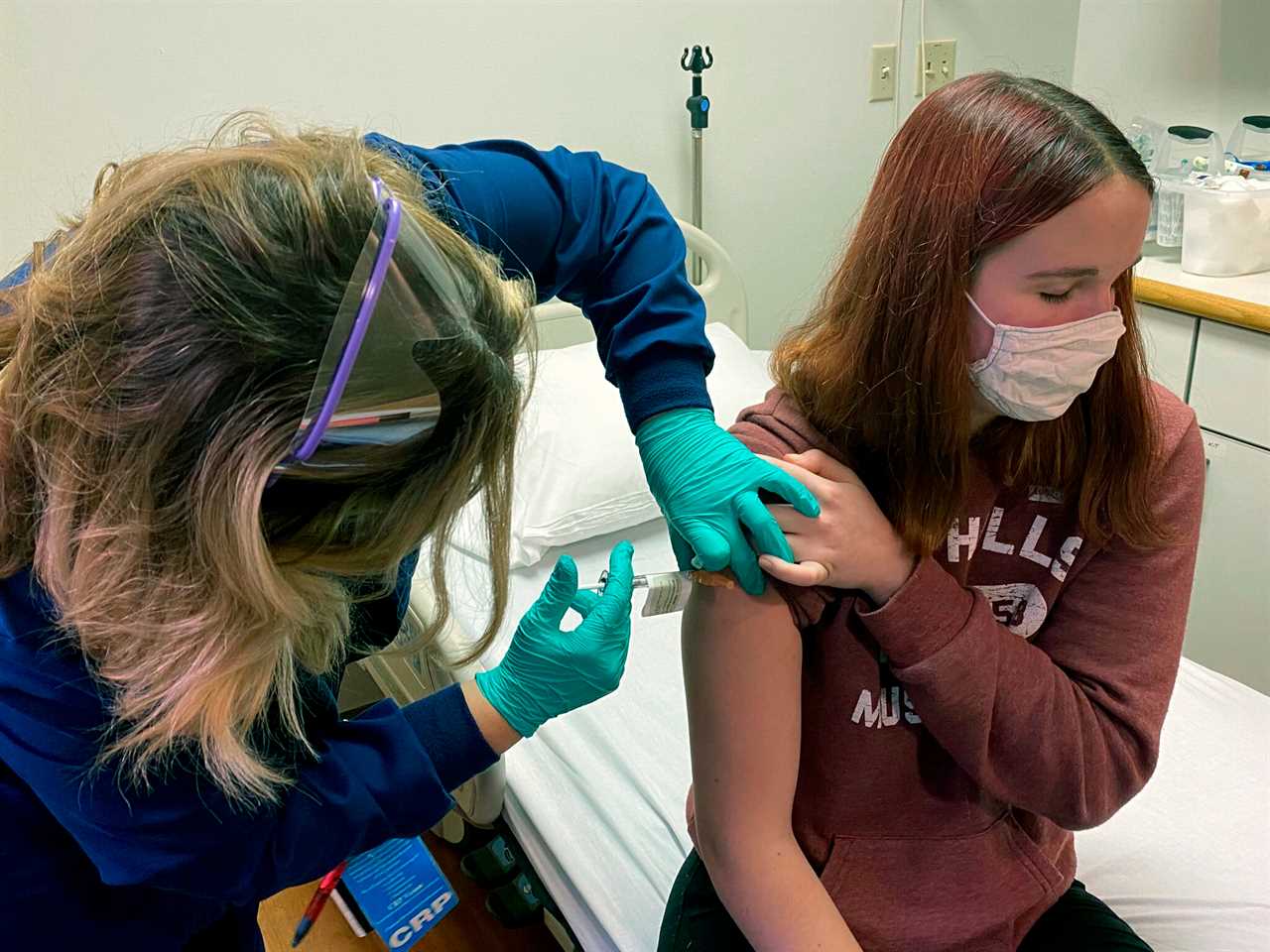
The vaccine won a key FDA endorsement, paving the way toward an official emergency use authorization (EUA) as soon as this week and possible shipment of the second major US coronavirus vaccine by this weekend.
The FDA released Moderna’s 30,000-person clinical study of its vaccine, along with the agency’s own analysis.
In a report on Tuesday, the FDA confirmed Moderna’s earlier assessment that the two-shot regimen was 94.1 percent effective against the coronavirus with minimal side effects and no safety concerns.
“[The] FDA has determined that the Sponsor [Moderna] has provided adequate information to ensure the vaccine’s quality and consistency for authorization of the product under an EUA,” the document read.
The agency’s vaccine panel (Vaccines and Related Biological Products Advisory Committee), consisting of independent scientists and advisors, is set to meet on Thursday to review Moderna’s product in detail. The same panel recommended Pfizer’s vaccine to the FDA last Thursday, and an official EUA was signed within 24 hours.
The data it will consider echoes the evidence that led to a 17 to 4 vote to authorize the Pfizer-BioNTech vaccine, which was 95 percent effective, The Washington Post reports.

Moderna’s documents posted by the FDA included new data suggesting that its vaccine begins to prevent asymptomatic infections after the first dose. The ability of a vaccine to prevent asymptomatic infections is important because it could help significantly slow the spread of the coronavirus.
In addition, Moderna’s vaccine was particularly effective against severe disease.
The FDA’s analysis of Moderna’s study found the most common side effects included injection-site pain, fatigue, headache and chills
The US plans to ship just under six million doses of Moderna vaccine once the FDA approves emergency use, Gustave Perna, chief operating officer of the White House’s Operation Warp Speed program, said on Monday.
“It will be a very similar cadence that was executed this week with Pfizer, where we’re hitting initial sites on Monday, follow on Tuesday and Wednesday,” Perna said.
Nearly three million doses of Pfizer’s vaccine have been distributed since Sunday.


The first shipments arrived at 145 distribution centers on Monday, while 425 more sites should get the vaccine on Tuesday and the final 66 sites on Wednesday.
Health care workers and nursing home residents are first in line to receive the shots.
The biggest vaccination effort in US history comes as the country has seen nearly 300,000 coronavirus deaths and exceeded 16million cases.
Did you miss our previous article...
https://trendinginthenews.com/covid-19/moment-scots-jet-ski-romeo-arrives-on-isle-of-man-after-45hr-journey-to-see-girlfriend-with-only-10-mins-of-fuel-left






