KIDS aged five to 11 are set to have their coronavirus vaccines as the European Union (EU) approves jabs for youngsters.
These kids will be given a lower dosage than kids aged 12 years and above after a study showed the immune response was similar to that of a higher dose.
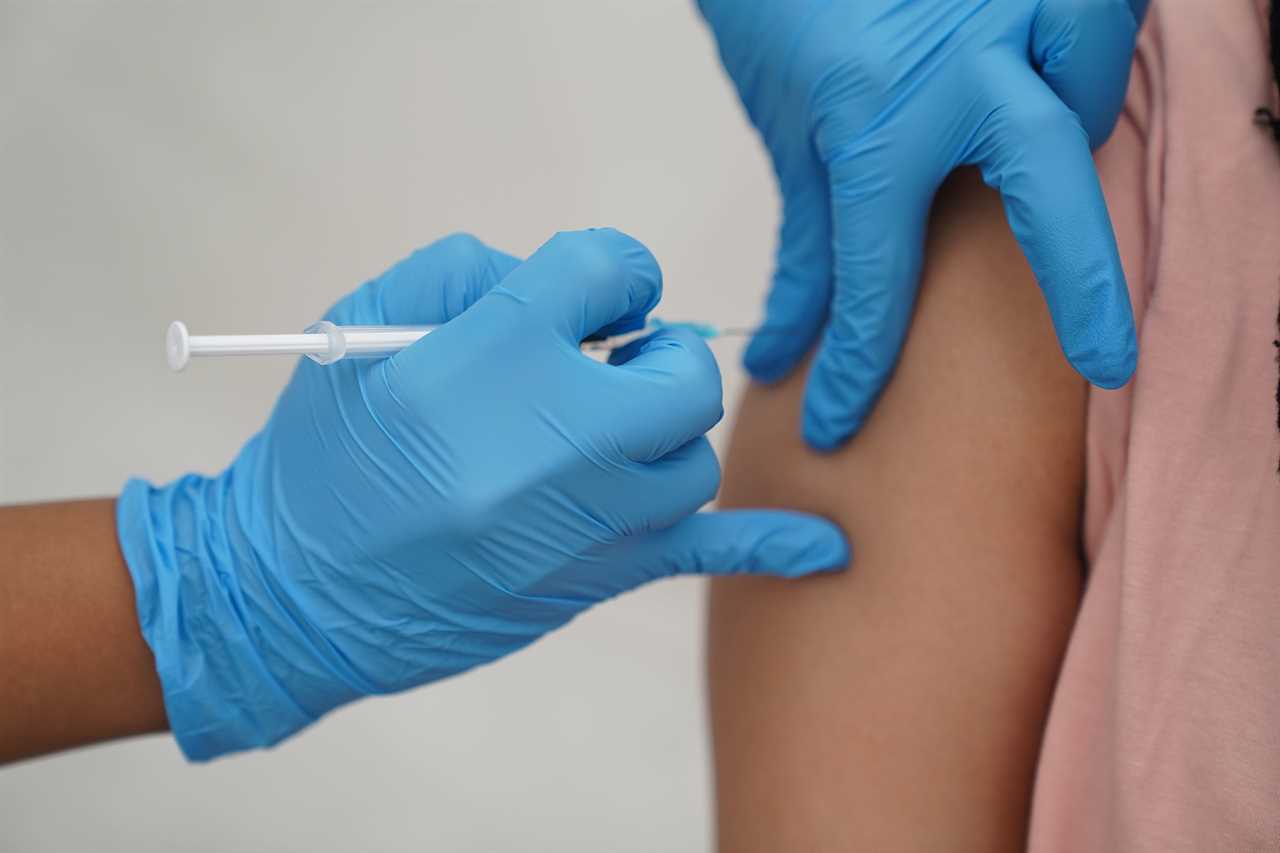
It means that British kids could get their jabs in a matter of weeks as UK regulators could follow the European Medicines Agency’s (EMA) recommendation.
The vaccine has been developed by Pfizer/BioNTech and it’s official name is Comirnaty.
The approval of the jab comes as coronavirus cases are continuing to rise in Europe.
A new study revealed Europe could face “300,000 deaths and one million people in hospital” as Covid wreaks havoc across the continent this winter.
The London School of Hygiene and Tropical Medicine study warned that 280,000 people alone could end up in hospital in Germany as Europe faces down the barrel of a new wave of Covid lockdowns.
In the UK, cases have also been rising in school-aged children, with experts in the most infected parts of the country claiming a rise in cases is down to kids and those who are not yet vaccinated.
The EMA tested the vaccine on 1,305 kids aged five to 11 who had no previous sign of coronavirus infection.
During the trial kids received either a vaccine or a placebo drug.
Of the 1,305 children receiving the vaccine, three developed Covid-19 compared to 16 out of the 663 children who received placebo.
It means that the vaccine was shown to be 90.7 per cent effective at preventing symptomatic Covid-19.
Experts noted that the true rate of prevention could be as low as 67.7 per cent and as high as 98.3 per cent.
The study found that the most common side effects in kids was similar to the general population.
While side effects were mild or moderate, the most common was pain in the site of injection, tiredness, headache, redness and swelling at the site of injection, muscle pain and chills.
These usually go away with a few days after vaccination.
The EMA’s human medicines committee said that the study showed that the benefits of the vaccine far outweighed the risks.
They said that this was particularly the case in children who had conditions that put them at risk of severe Covid-19.






