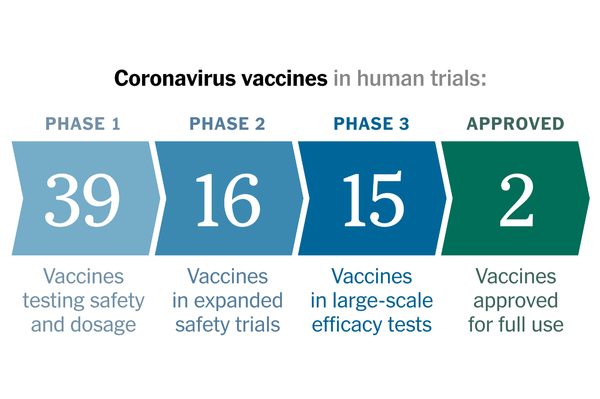
The Food and Drug Administration authorized Pfizer’s Covid-19 vaccine for emergency use on Friday, according to three people with knowledge of the decision who spoke on condition of anonymity because they were not authorized to discuss it. The action means millions of highly vulnerable people will begin receiving the vaccine within days.
The authorization is a historic turning point in a pandemic that has taken more than 290,000 lives in the United States. With the decision, the United States becomes the sixth country — in addition to Britain, Bahrain, Canada, Saudi Arabia and Mexico — to clear the vaccine. Other authorizations, including by the European Union, are expected within weeks.
The action followed an extraordinary sequence of events on Friday morning when the White House chief of staff, Mark Meadows, told the F.D.A. commissioner, Dr. Stephen Hahn, to consider looking for his next job if he didn’t get the emergency approval done on Friday, according to a senior administration official who spoke on condition of anonymity because he was not authorized to discuss the matter. Dr. Hahn then ordered vaccine regulators at the agency to do it by the end of the day.
The authorization set off a complicated coordination effort from Pfizer, private shipping companies, state and local health officials, the military, hospitals and pharmacy chains to get the first week’s batch of about three million doses to health care workers and nursing home residents as quickly as possible, all while keeping the vaccine at ultracold temperatures.
Pfizer has a deal with the U.S. government to supply 100 million doses of the vaccine by next March. Under that agreement, the shots will be free to the public.
Every state, along with six major cities, has submitted to the federal government a list of locations — mostly hospitals — where the Pfizer vaccine is to ship initially. In populous Florida, the first recipients will be five hospitals, in Jacksonville, Miami, Orlando, Tampa and Hollywood. In tiny, rural Vermont, only the University of Vermont Medical Center and a state warehouse will get supplies.
McKesson Corporation, a giant medical supplier, is sending kits of syringes, alcohol pads, face shields and other supplies to the same sites, where they will meet up with the vaccines that Pfizer is shipping in special boxes, packed with dry ice, designed to keep them at minus 94 degrees Fahrenheit.
The Pfizer packaging will include a device that tracks the location of the box, plus a thermal probe that will make sure the deep freeze is maintained throughout the journey from the company’s distribution sites in Michigan and Wisconsin.
The decision is a victory for Pfizer and its German partner BioNTech, which began working on the vaccine 11 months ago. Vaccines typically take years to develop. The companies’ late-stage clinical trial, which enrolled nearly 44,000 people, was found to be 95 percent effective.
An expert panel advising the F.D.A. on Thursday gave its approval of Pfizer’s vaccine for people 16 and older, and the agency was planning to release the formal authorization on Saturday. That timeline was accelerated by half a day after President Trump attacked Dr. Hahn for failing to authorize a vaccine more quickly. But the accelerated announcement was not expected to speed up the delivery of vaccines around the country.
Mr. Trump told Dr. Hahn on Twitter on Friday morning to “stop playing games and start saving lives!!!” He called the F.D.A. “a big, old, slow turtle,” flush with funds but mired in bureaucracy.
Mr. Trump has repeatedly accused the F.D.A. and the drugmakers themselves of slow-walking the approval process in order to harm him politically. Allies of Dr. Hahn have been on tenterhooks for weeks, expecting him to be fired any day.
The president wrote that with “my pushing,” the administration had shaved years off the development of vaccines. “Get the dam vaccines out NOW, Dr. Hahn,” he wrote, misspelling the expletive.
The threat to Dr. Hahn’s job was first reported by The Washington Post. In a statement, Dr. Hahn denied that Mr. Meadows told him he should consider seeking another job, calling it “an untrue representation of the phone call." Instead, Dr. Hahn said, his agency was “encouraged to continue working expeditiously.”
Even though the F.D.A. was going to approve the Pfizer vaccine in any case, some experts warned that the pressure from the White House could undermine public trust in the agency’s decision-making.
“This may actually do more harm than good, because all it will do is inject more politics into a scientific process,” said Dr. Aaron S. Kesselheim, a professor at the Brigham and Women’s Hospital and Harvard Medical School.
A similar vaccine, developed by Moderna, is also under review by the F.D.A. and could soon be cleared for emergency use. On Friday, the federal government announced it had ordered another 100 million doses from Moderna, adding to a deal this summer for an initial supply of 100 million doses. Other vaccines, including ones developed by Johnson & Johnson and AstraZeneca, are in late-stage trials and could be authorized in the next few months.
In anticipation of the vaccine arriving across the country, Americans expressed both hope and anxiety.