WASHINGTON — The Food and Drug Administration on Saturday authorized Johnson & Johnson’s single-shot Covid-19 vaccine for emergency use, beginning the rollout of millions of doses of a third effective vaccine that could reach Americans by early next week.
The announcement arrived at a critical moment, as the steep decline in coronavirus cases seems to have plateaued and millions of Americans are on waiting lists for shots.
Johnson & Johnson has pledged to provide the United States with 100 million doses by the end of June. When combined with the 600 million doses from the two-shot vaccines made by Pfizer-BioNTech and Moderna slated to arrive by the end of July, there will be more than enough shots to cover any American adult who wants one.
But federal and state health officials are concerned that even with strong data to support it, some people may perceive Johnson & Johnson’s shot as an inferior option.
The new vaccine’s 72 percent efficacy rate in the U.S. clinical trial site — a number scientists have celebrated — falls short of the roughly 95 percent rate found in studies testing the Moderna and Pfizer-BioNTech vaccines. Across all trial sites, the Johnson & Johnson vaccine also showed 85 percent efficacy against severe forms of Covid-19 and 100 percent efficacy against hospitalization and death.
“Don’t get caught up, necessarily, on the number game, because it’s a really good vaccine, and what we need is as many good vaccines as possible,” Dr. Anthony S. Fauci, the government’s top infectious disease expert, said in an interview on Saturday. “Rather than parsing the difference between 94 and 72, accept the fact that now you have three highly effective vaccines. Period.”
If Johnson & Johnson’s vaccine would have been the first to be authorized in the United States instead of the third, “everybody would be doing handstands and back flips and high-fives,” said Dr. James T. McDeavitt, dean of clinical affairs at the Baylor College of Medicine.
On Sunday a committee of vaccine experts who advise the Centers for Disease Control and Prevention will meet to discuss whether certain population groups should be prioritized for the vaccine, guidance that state health officials have been eagerly awaiting in anticipation of the F.D.A.’s authorization.
One administration official familiar with the distribution of the vaccine said that shipments would begin on Monday and deliveries could arrive as soon as Tuesday.
Johnson & Johnson has said it will ship nearly four million doses as soon as the F.D.A. authorizes distribution and another 16 million or so doses by the end of March. That is far fewer than the 37 million doses called for in its $1 billion federal contract, but the contract says that deliveries that are 30 days late will still be considered timely.
The federal government is paying the firm $10 a dose for a total of 100 million doses to be ready by the end of June, substantially less per dose than it agreed to pay Moderna and Pfizer, which developed its vaccine with a German partner, BioNTech.
Johnson & Johnson’s one-dose vaccine will allow states to rapidly increase the number of people who have been fully inoculated. Unlike the other two vaccines, it can be stored at standard refrigeration temperatures for at least three months.
Dr. Danny Avula, the vaccine coordinator for Virginia, said the Johnson & Johnson shipments would boost the state’s allotment of vaccine next week by nearly one-fifth.
“I’m super-pumped about this,” he said. “A hundred percent efficacy against deaths and hospitalizations? That’s all I need to hear.”
He said the state was planning mass vaccination events specifically for the Johnson & Johnson vaccine, partly to quell any suspicion that it is a lesser product targeted to specific groups.
“It will be super clear that this is Johnson & Johnson, here’s what you need to know about it. If you want to do this, you’re coming in with eyes wide open,” he said. “If not, you will keep your place on the list.”
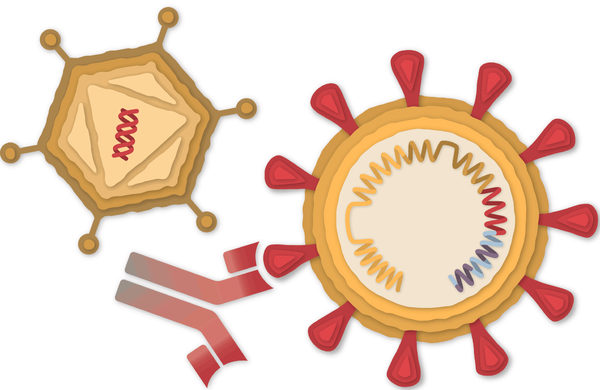
How the Johnson & Johnson Vaccine Works
An adenovirus helps prime the immune system to fight the coronavirus.
Michele Roberts, the assistant secretary of Washington State’s health department, said that it would be difficult to explain the technical aspects of how Johnson & Johnson’s vaccine trials differed from those of other drug makers. Because the studies were conducted at different times and with different protocols, precise comparisons can be problematic. All three trials showed the vaccines provided strong protection against Covid-19, especially for severe disease.
Latest Updates
- New Zealand’s largest city enters its second lockdown in February.
- North Carolina will send 3,500 prisoners home early to avoid the virus.
- Tech giants ban the sale of N95 masks, citing rules that scientists say are outdated.
Understanding the subtle contrasts requires a lot of “scientific literacy,” she said. “There are so many different factors at play. But those aren’t, you know, quick public messages.”
Even some clinicians misinterpret the differences among the Covid-19 vaccines, health officials said. “They assume it’s apples to apples but it’s apples to oranges, or worse, apples to tires,” said Dr. Nirav Shah, the director of the Maine Center for Disease Control and Prevention.
Last week, Dr. Shah said, the leader of one group of specialty health clinics in his state initially turned down his offer to ship doses of the Johnson & Johnson vaccine, saying his health practitioners were concerned it was less efficacious than the other two.
He said he told him: “Stop right there. We need to have a Zoom conversation right now with your entire medical staff.” Instead, he carefully explained Johnson & Johnson’s results to the provider, who then spoke with his staff. Twenty minutes later, the provider sent him a message saying: “We’re on board. Send us the J & J.”
Some state officials have been frustrated by what they view as a lack of a coordinated plan from the Biden administration on how to deploy the new vaccine. Governors have asked the White House for guidance, but administration officials have so far left it up to the states to decide.
Even though Johnson & Johnson received ample federal support and agreed to manufacture at risk, federal officials familiar with its operation said the company took an overly conservative approach to production, emphasizing scaling up on the back end of its contract.