SCIENTISTS have spent months racing against the clock to create the first coronavirus vaccine in a bid to save millions of lives.
And now, as the number of coronavirus deaths in the UK nears 55,000, a world-leading Pfizer vaccine could be ready for use as soon as mid-December.

When will a coronavirus vaccine be available in the UK?
Best case scenarios see the vaccine being rolled out before Christmas but more conservative guesses predict it will be some time next year.
Leaked documents recently revealed that every adult in the UK will be vaccinated against Covid by April under radical NHS plans to bring an end to the pandemic.
On Friday, November 20, Health Secretary Matt Hancock said the first Brit patients could get a vaccine in December, subject to approval.
Leaked plans, seen by Health Service Journal (HSJ), suggest health bosses are primed to immunise a record 44 million people within five months of a jab being available.
Under draft proposals vaccination will start in early December, depending on regulatory approval in the US.
The ambitious provisional timetable sets out plans to protect the nation at breakneck speed – with five million jabs doled weekly.
The NHS hopes to finish immunising 20 million high-risk Brits, such as care home residents and frontline health workers, by late February.
Deputy chief medical officer Professor Jonathan Van-Tam also said he was hopeful “that we could begin to see some vaccine by Christmas”.
Prof Van-Tam said at a press briefing in November: “We might be able to look towards the end of spring for a much better horizon than we have in front of us right now.”
Everyone aged 18 to 50 will also be eligible for a jab from early 2021, with the programme aiming to wrap up by late April.
However, Dr Onyema Ogbuagu, who is serving as principal investigator for Pfizer’s vaccine clinical trials, said: “I think that the most optimistic expectations around the vaccine availability would be the first quarter of 2021.”
It took just ten months for the Pfizer vaccine to be developed.
In contrast, a traditional jab would take between ten to 15 years to develop.
Drug regulators must now wait for safety data released later this month before they can grant emergency approval for widespread use.
Boris Johnson told a Downing Street press conference on November 9 that the UK is ready to roll out the jab when it gets the green light from regulators.
He said: “Earlier this year the UK Government ordered 40 million doses of the Pfizer vaccine – enough for about a third of the population, since you need two doses each.
“That puts us towards the front of the international pack on a per capita basis – and I should add we’ve ordered over 300 million doses from five other vaccine candidates as well.
“If the Pfizer vaccine passes all the rigorous safety checks and is proved to be effective then we will begin a UK-wide NHS led programme of vaccine distribution.”
Have vaccine human trials begun in the UK?
More than 300 clinical trials for a Covid-19 vaccines occurring around the world with about 12 currently in the final stages of testing, with Pfizer the first to report any results.
Volunteers in the US, who have tested the Pfizer jab have said it feels like a “severe hangover”.
In the UK, a jab developed by Oxford University began human trials in mid-April – with the top university receiving a £20million boost.
An Oxford consortium is testing its vaccines in 6,000 healthy volunteers, including healthcare workers.
The Oxford study is tracking about 1,000 people, half of whom have been given the real vaccine.
But the team plans a later-stage study with another 5,000 volunteers for a final answer and expects it to be tested in other countries.
Imperial College, meanwhile, is involved in two clinical trials testing a vaccine against Covid-19, led by Professor Robin Shattock.
It’s joined the Oxford-led clinical study, with trials conducted at multiple centres across the UK, including Oxford, Southampton, Bristol and London.
Imperial will be one of several sites carrying out trials of the vaccine, which is being fast-tracked by £22.5million of government funding.
In September 2020, Sanofi and GSK vaccine announced the start of human trials, followed by a Phase 3 study in December 2020.
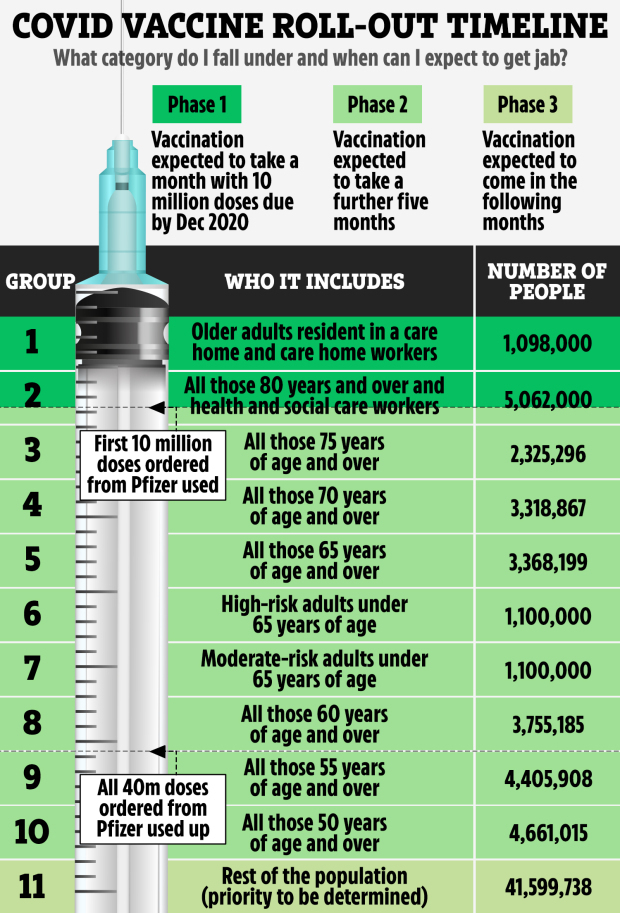
Who will get Covid vaccine first?
The first people to get the vaccine will be frontline heath and social care workers, the elderly and the most vulnerable.
The first mass vaccination centres are planned for sites in major cities including Leeds, Hull and London.
They will be supported by hundreds of mobile vaccination units dotted nationwide, while roving teams will visit care homes and vulnerable Brits.
The Joint Committee on Vaccination and Immunisation (JCVI) has examined data on who suffers the worst outcomes from coronavirus and who is at highest risk of death. Its interim guidance says the order of priority should be:
- Older adults in a care home and care home workers All those aged 80 and over and health and social care workers, though they may move up the list Anyone 75 and over
- People aged 70 and over
- All those aged 65 and over
- High-risk adults under 65
- Moderate-risk adults under 65
- All those aged 60 and over
- All those 55 and over
- All those aged 50 and over
- The rest of the population, with priority yet to be determined.

How effective is the vaccine?
Pfizer has said that its jab was proven to be 90 per cent effective at preventing Covid – on what experts hailed a “great day for humanity”.
The pharmaceutical company said interim findings from its major clinical trial shows that nine out of 10 people who had the jab were protected from Covid-19.
Sir John Bell, professor of medicine at Oxford University, said the news means “life will return to normal by spring”.
Prof Brendan Wren, from the London School of Hygiene & Tropical Medicine, said: “A 90 per cent efficacy for a phase 3 trial is excellent for a new vaccine that could make a huge difference, but more confirmatory safety and efficacy studies are required.
“The RNA-based vaccine requires two doses and its true efficacy over a longer period of time remains to be evaluated. These are encouraging results and it is a case of so far so good.”
How long will the vaccine work for?
The jab is designed to prevent people developing Covid-19 and is administered in two shots about three weeks apart.
Experts behind the mRNA-based vaccine say they are “optimistic” it could offer protection from Covid-19 for “at least a year”.
Although they can’t say with certainty how long the immunisation effect will last, research on recovered patients have led them to believe it won’t be short-lived.
Ugur Sahin, chief executive of BioNTech which has co-developed the jab with Pfizer, said: “We should be more optimistic that the immunisation effect can last for at least a year”.






