A MASS coronavirus vaccination programme kicked off in the UK this week – offering hope that the end of the pandemic is in sight.
The first person to receive the Pfizer Covid jab in Britain was 90-year-old gran Margaret Keenan who urged others to follow her lead.
Some people aren’t allowed to have the new Covid vaccine
It’s since been rolled out at 50 NHS hospital hubs across the country – with thousands of patients and staff now immunised.
The Government is yet to publish exactly how many people have had the jab but says information on numbers will be available in due course.
It comes as the medicines regulator issued new advice after two NHS staff with a history of severe allergies fell ill after having the Covid jab on V-Day.
Those who are most at risk from coronavirus are being encouraged to get the jab.
This includes people over 80 who are in hospital or have an appointment in the coming weeks, care home workers and frontline NHS staff.

Margaret Keenan, 90, being discharged from hospital after being the first person to get the Covid jab
It’s hoped the vaccine will be offered more widely – and at other locations – as soon as possible.
The order in which people will be offered the vaccine is based on advice from the Joint Committee on Vaccination and Immunisation (JCVI).
But there are three groups of people who aren’t allowed the jab…
1. People with severe allergies
People with a history of life-threatening allergic reactions to a vaccine or food should not get the Pfizer Covid-19 jab, the head of the UK’s medicines regulator said.
But June Raine, chief executive of the Medicines and Healthcare products Regulatory Agency (MHRA), said anaphylaxis was a “known … very rare side effect with any vaccine”.
It comes after two NHS staff members who received the jab on Tuesday had allergic reactions after being given the Pfizer/BioNTech vaccine.
The health workers, who are understood to both have a history of severe allergic reactions, were among thousands to receive the vaccine on the first day of the Covid-19 mass vaccination programme.
A further report of a possible allergic reaction following immunisation was also received by the MHRA.
Dr Raine said a group including experts on allergy and clinical immunology was convened on Wednesday to consider any possible mitigation to the “rare risk of anaphylaxis”.
She said: “Any person with a history of anaphylaxis to a vaccine, medicine or food should not receive the Pfizer BioNTech vaccine. A second dose should not be given to anyone who has experienced anaphylaxis following administration of the first dose of this vaccine.
“Anaphylaxis is a known, although very rare, side effect with any vaccine. Most people will not get anaphylaxis and the benefits in protecting people against Covid-19 outweigh the risks.”
Pfizer said the vaccine was “well tolerated” during the trials with “no serious safety concerns”.
Dr Raine added: “You can be completely confident that this vaccine has met the MHRA’s robust standards of safety, quality and effectiveness.
“The safety data has also been critically assessed by the government’s independent advisory body, the Commission on Human Medicines. No vaccine would be approved unless it meets these stringent standards – on that you can be sure.”
2. Pregnant women
Pregnant women are being advised to wait until they’ve had their baby to get the Covid jab.
Those who are trying to get pregnant, should also wait for two months after having the second dose before getting pregnant, according to the NHS.
New mums should also wait until they have finished breastfeeding before having the jab, health officials advise.
If you were breastfeeding when you had the first dose you are advised not to have the second dose until you have finished.
The Department of Health says: “The Pfizer-BioNTech Covid-19 vaccine is a new type of vaccine that has been shown to be effective and to have a good safety profile.
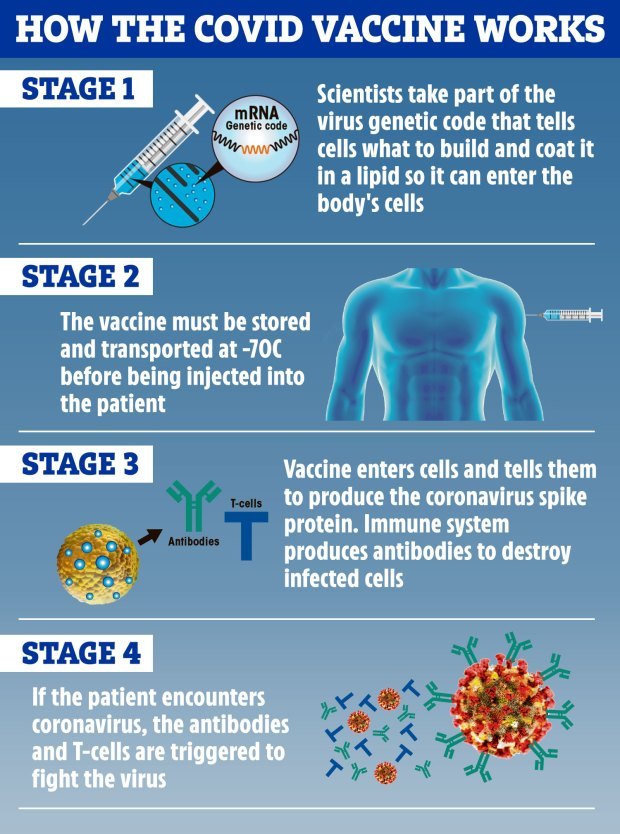
“It has not yet been assessed in pregnancy, so it has been advised that until more information is available, those who are pregnant should not have this vaccine.”
Deputy chief medical officer for England, Professor Jonathan Van-Tam, said none of the vaccine trials deliberately included pregnant women, which is why there is a lack of information on the effects of the jab on this group.
Due to this, the JCVI has advised a precautionary approach and says women should not come forward for the jab if pregnant or planning a pregnancy within three months of the first dose.
More data is expected and these measures will be reviewed as soon as it becomes available.
3. Children under 16
Almost all children who are infected with Covid-19 will have asymptomatic infection or mild disease.
At this time there is very limited data on vaccination in adolescents, with no information on younger children – as they were not included in clinical trials.
The Joint Committee on Vaccination and Immunisation (JCVI) advises that only those children at very high risk of exposure or serious outcomes – such as older children with severe neuro-disabilities that require residential care – should be offered vaccination.
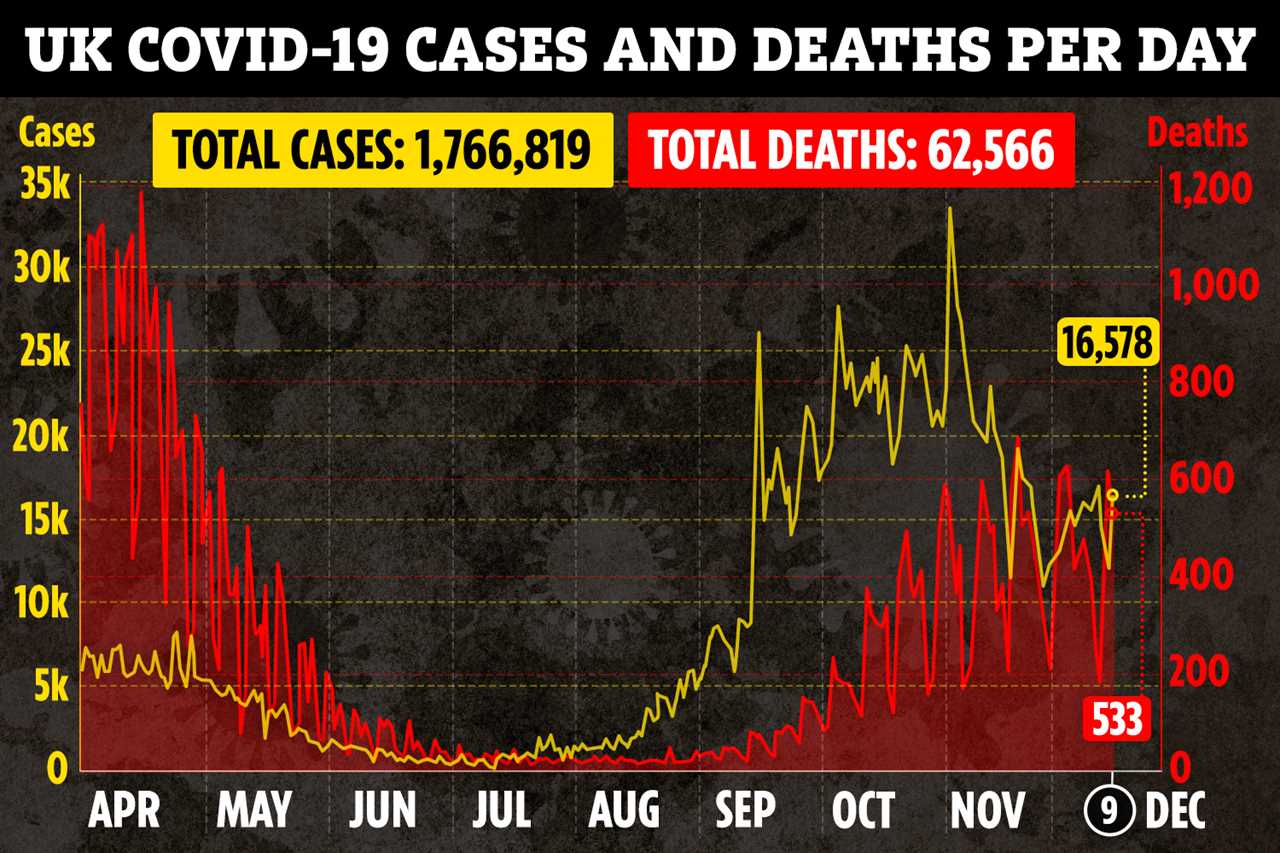
It says that clinicians should discuss the risks and benefits of vaccination with a person with parental responsibility.
They should be informed about the lack of safety data on the vaccine in children under the age of 16, the committee says.
The JCVI adds: “As trials in children and pregnant women are completed, we will also gain a better understanding of the safety and effectiveness of the vaccines in these persons.”










