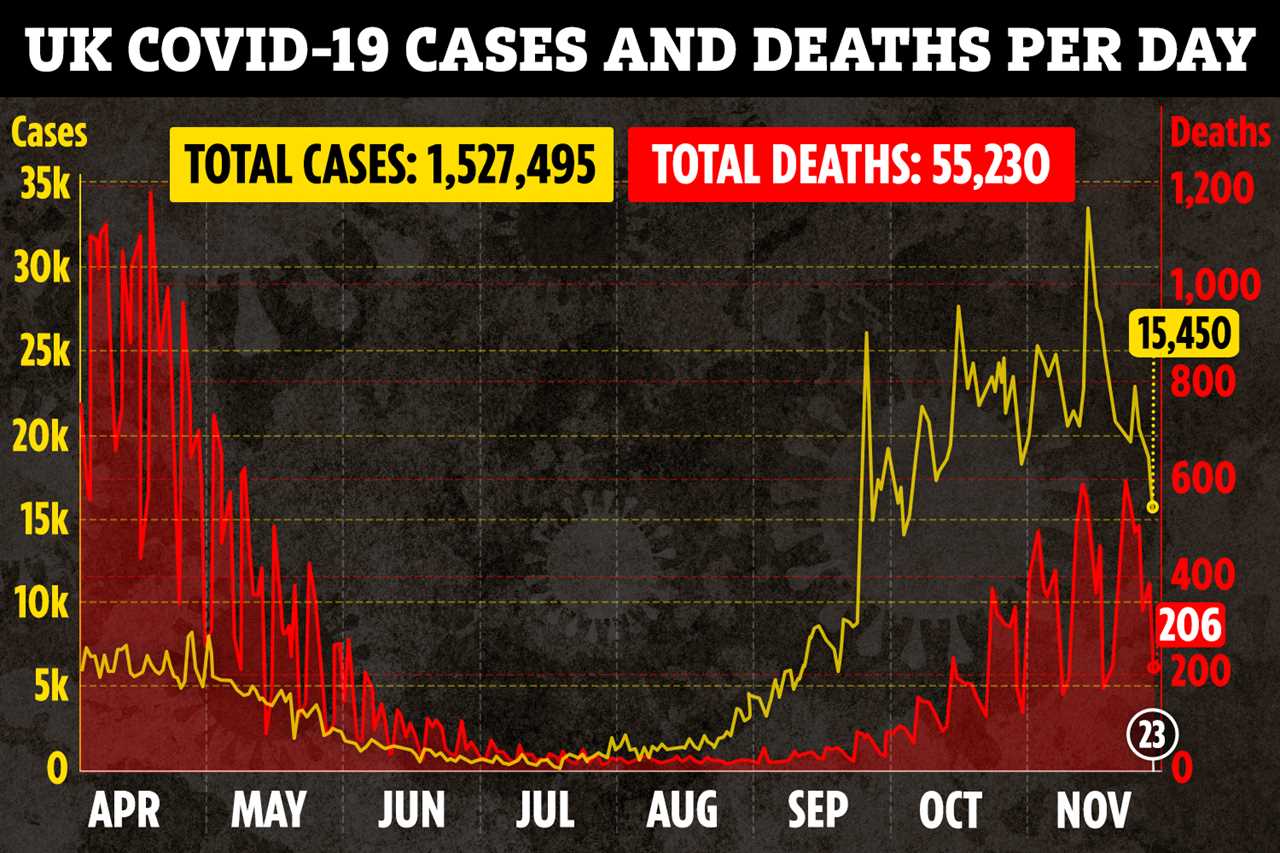
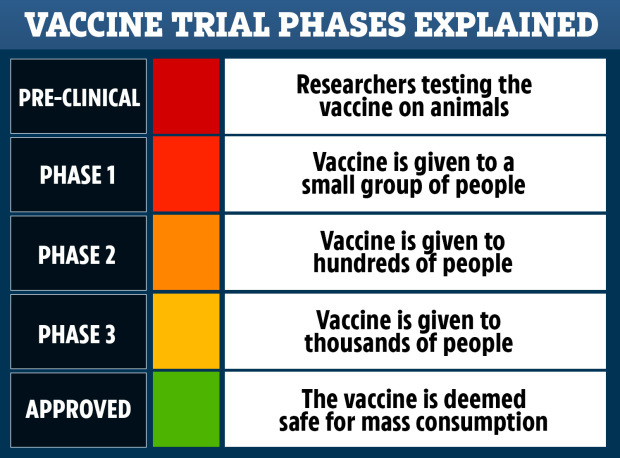
THE high effectiveness of the Oxford Covid vaccine is down to luck after a mistake in trials, its maker has admitted.
Trial data found giving participants two full doses resulted in 62 per cent protection against Covid-19.
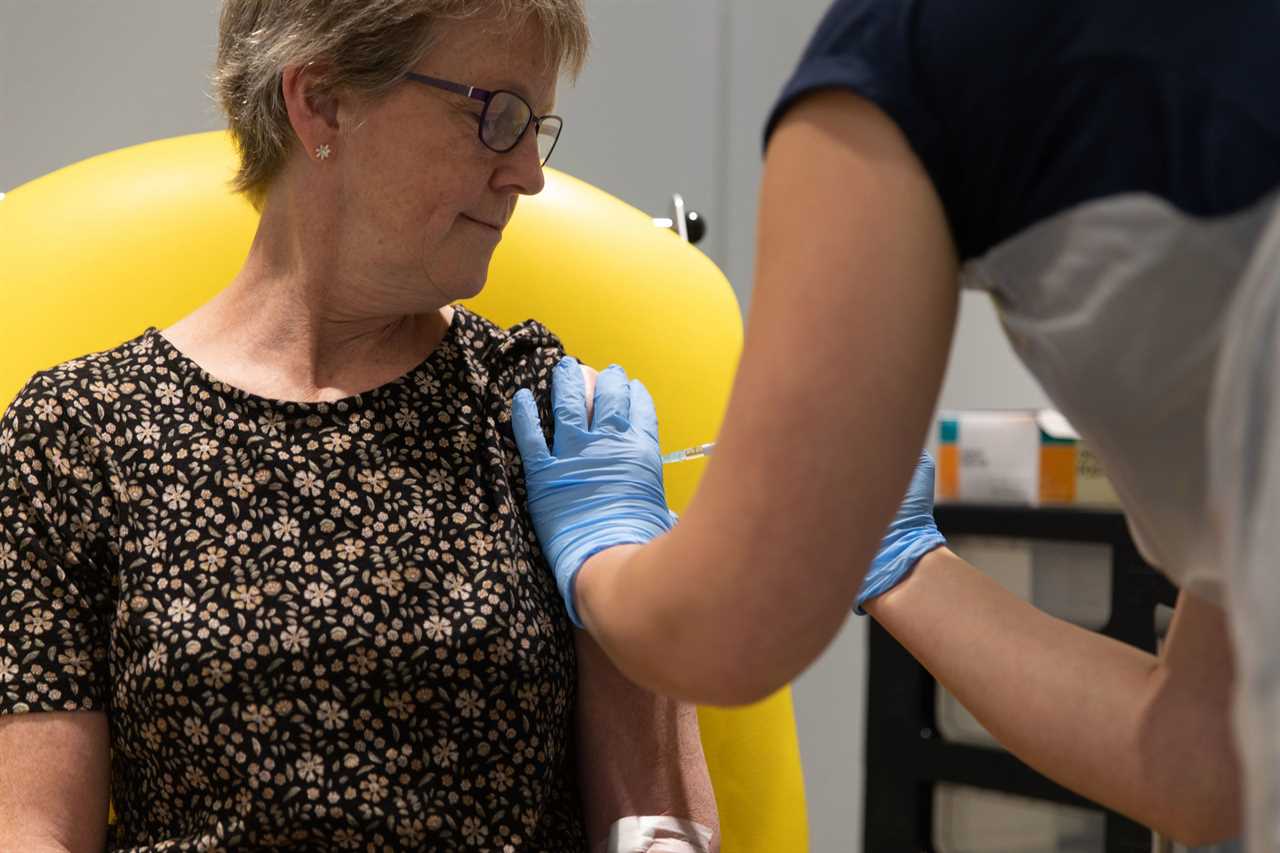
However, an initial half dose – followed up by a full dose a month later – was 90 per cent effective.
Only requiring a half dose of the vaccine will mean millions more Brits than first expected could be able to get a Covid jab, provided the Oxford proposal gets the go-ahead.
Experts think first priming the immune system with a weaker jab, before delivering a booster works better as it stops the body fighting back against the vaccine.
But Mene Pangalos, executive vice president at AstraZeneca, said the discovery was down to pure luck.
He said: “The reason we had the half-dose is serendipity.”
When the trial started in April, university researchers began administering doses to Brit volunteers.
But some experienced much milder symptoms.
He added: “So we went back and checked… and we found out that they had underpredicted the dose of the vaccine by half.”
However, they decided to continue the trial despite the initial error.
Britain has pre-ordered 100 million doses of the Oxford jab – which is expected to cost just £2 a time and can be stored at standard temperatures – with four million ready to be rolled out as soon as it gets regulatory approval.
CHEAP AND EASY
The Oxford/AstraZeneca vaccine is cheaper and easier to distribute than the US’s Pfizer and Moderna vaccines, which both revealed similarly promising results of around 95 per cent effectiveness last week.
It has also been shown to work in different age groups, including the elderly, and there were no hospitalised or severe cases in anyone who received the jab.
The preliminary data shows the vaccine works nine in ten times when it’s first given as a half dose, then followed by a full dose a month later.
It’s not clear why, but the team think it could be that a smaller dose may be a better way of kicking the immune system into action.
The effectiveness drops to 62 per cent when given as two full doses at least one month apart, to give a combined average efficacy of 70 per cent – which experts say is more than most flu jabs.
Health Secretary Matt Hancock said that the Medicines and Healthcare products Regulatory Agency (MHRA) would now assess if the 90 per cent effectiveness dosing regime could be used.
He told BBC Breakfast: “I’m really very pleased, I really welcome these figures – this data that shows that the vaccine in the right dosage can be up to 90 per cent effective.

“Of course, it’s vital that the independent regulator, the MHRA, will need to look at the data, will need to check to make sure that it’s effective and safe of course.
“But we’ve got 100 million doses on order and, should all that go well, the bulk of the rollout will be in the new year.”
Mr Hancock added that Brits could expect to see life return to normal by Easter next year.
Prime Minister Boris Johnson tweeted: “Incredibly exciting news the Oxford vaccine has proved so effective in trials. There are still further safety checks ahead, but these are fantastic results. Well done to our brilliant scientists at @UniofOxford & @AstraZeneca, and all who volunteered in the trials.”
The vaccine – called ChAdOx1 nCoV-19 – uses a harmless, weakened version of a common virus which causes a cold in chimpanzees.
Unlike the Pfizer vaccine – which has been found to be 95 per cent effective – the Oxford jab can be stored at more standard fridge temperatures.
Prof Andrew Pollard, chief investigator of the Oxford trial, said: “These findings show that we have an effective vaccine that will save many lives.
“Excitingly, we’ve found that one of our dosing regimens may be around 90 per cent effective and, if this dosing regime is used, more people could be vaccinated with planned vaccine supply.”
Prof Pollard told the BBC Radio 4 Today programme that it was important to begin mass vaccinations as soon as possible.
“The most important thing to get us back to normal is to use these vaccines – all of the vaccines that are going to be available – as soon as possible,” he said.
“Once we have protected the vulnerable in the population we will be able to start getting back to normal.”
“We have just got to get on with this as soon as possible.”
‘STOP VIRUS IN ITS TRACKS’
The results also revealed lower levels of asymptomatic infection in the smaller dose group, he said.
Prof Pollard added: “There is just a hint in the data at the moment that those who got that regime with higher protection, there is a suggestion that it was also able to reduce asymptomatic infection.
“If that is right, we might be able to halt the virus in its tracks and stop transmitting between people.”
Speaking later a press briefing, he said not enough time has passed to know if people are still protected from the virus a year after being vaccinated.