MIXING doses of the AstraZeneca and Pfizer vaccines appears safe, but may trigger more side effects.
According to the first study of its kind, taking a dose of both vaccines may make people feel more unwell than if they had just been given one type.

Read our coronavirus live blog for the latest updates
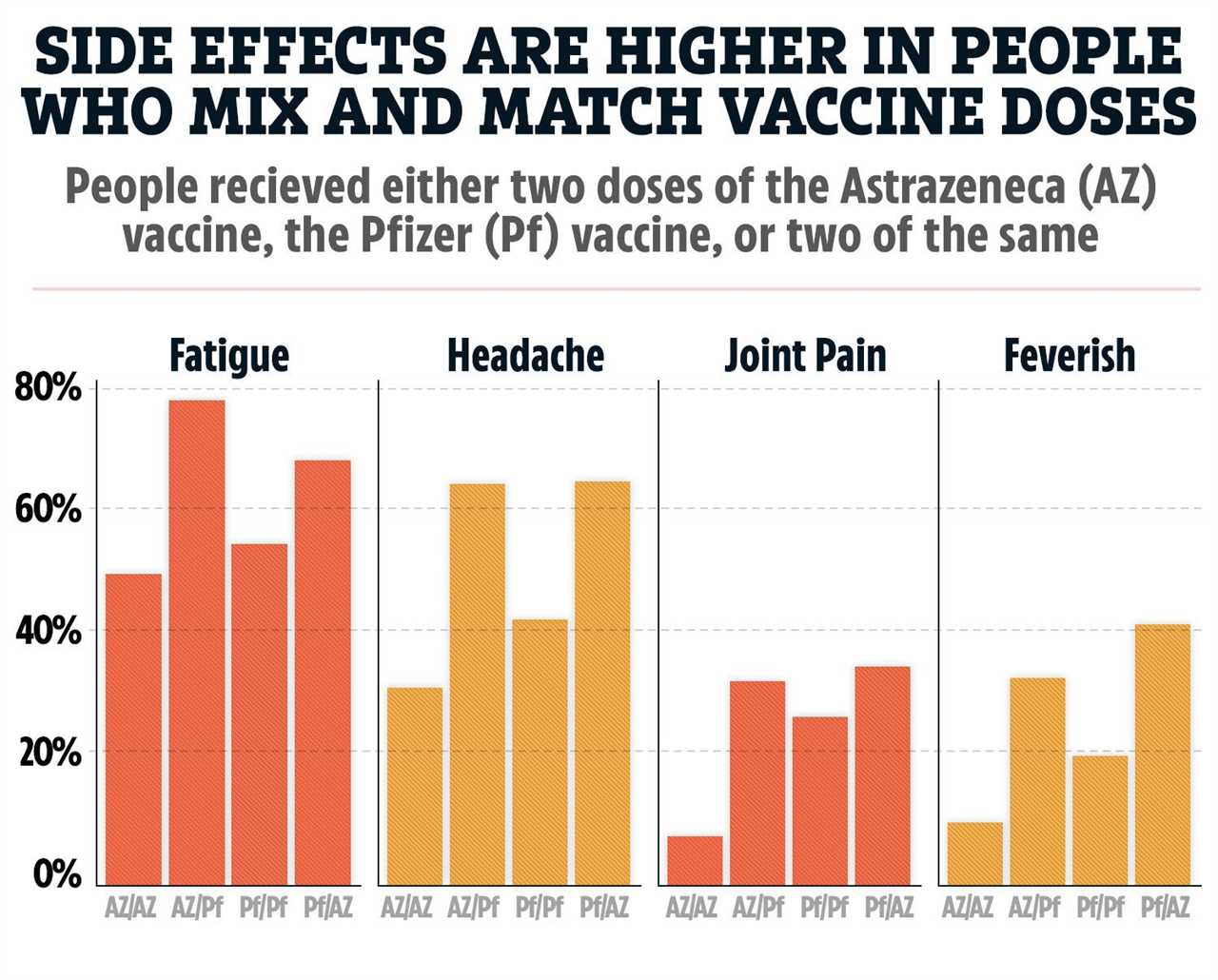
How some of the most common side effects compare in people who had mixed vaccine doses (AZ/PF or PF/AZ) or the same of one type (AZ/AZ or PF/PF)
But the effects are short-lived and last no more than 48 hours, the scientists at University of Oxford reassured.
Most Covid jabs require two doses at least 21 days apart, the first being called the “primer” and the second the “booster”.
The Com-COV study aimed to see whether two vaccines that work in very different ways can work in tandem.
It recruited 830 volunteers aged 50 and above across England to look at four different vaccine dose regimens:
- a first dose of the Oxford-AstraZeneca vaccine followed by boosting with either the Pfizer vaccine or a further dose of the Oxford-AstraZeneca vaccine
- or a first dose of the Pfizer vaccine followed by boosting with either the Oxford-AstraZeneca vaccine or a further dose of the Pfizer vaccine.
Participants were randomly assigned to one of the dosing groups and then reported how they felt in the seven days following their jab.
Some 41 per cent who had a Pfizer dose followed by a AZ dose reported feeling feverish.
This was double the 21 per cent who had two Pfizer doses.
Of those who had the AZ jab followed by the Pfizer one, 34 per cent reported a fever compared with 10 per cent of those whose doses were both the AZ jab.
Similar increases were observed for chills, fatigue, headache, joint pain, muscle ache and a general unwell feeling.
Because people who were given a cocktail of doses had more side effects, they were also more likely to take paracetamol, the study found.
Around 60 per cent turned to the pain-relieving drug compared with 36-41 per cent of those who had the same vaccine for each dose.
The preliminary data has not been published yet, but will be available in June of this year.
In a linked commentary published in the Lancet, researchers noted all participants were over the age of 50.
They predict that younger people will be even more likely to see increases in side effects.
Already younger people report more symptoms in the days after a jab, which scientists say is a result of a stronger immune reaction.
Prof Matthew Snape, Chief Investigator on the trial, said: “The results from this study suggest that mixed dose schedules could result in an increase in work absences the day after immunisation.”
He said this is important when planning the vaccination of healthcare workers specifically, of which there are already shortages of.
Prof Snape added: “Importantly, there are no safety concerns or signals, and this does not tell us if the immune response will be affected.
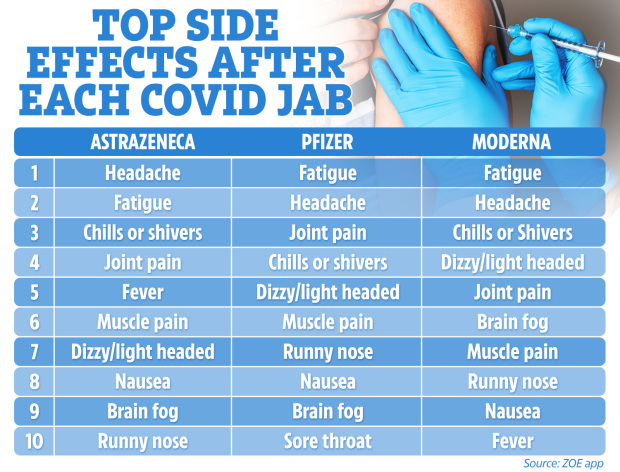 The most common side effects after a dose of either of the three vaccine doses given in the UK, according to the ZOE Covid Symptom Study app
The most common side effects after a dose of either of the three vaccine doses given in the UK, according to the ZOE Covid Symptom Study app
“We hope to report these data in the coming months. In the meantime, we have adapted the ongoing study to assess whether early and regular use of paracetamol reduces the frequency of these reactions.”
Blood clot links
The Com-COV study was launched earlier this year after extremely rare blood clots were linked to the AstraZeneca vaccine.
Although the AstraZeneca jab is still deemed extremely beneficial, some countries are restricting second doses of it.
Germany, France, Sweden, Norway and Denmark are among those that are pushing for mixed vaccines in those who have already had one dose of the AstraZeneca jab.
In the UK, everyone is being told to get their second dose of the AstraZeneca vaccine.
But it will no longer be given to those under the age of 40 who appear most at risk of the blood clots.
Combining vaccines would also be beneficial if stocks become limited in the future and thereby slow down programmes.
There is also the potential it creates even better protection against Covid, as Prof Snape has previously said there are early signs this could be the case.
He told Times Radio in February: “There’s some suggestions from animal studies in mice that actually you may get a better immune response if you, for example, combine the AstraZeneca-type vaccines with an RNA-type vaccine, and that actually seems to generate in some aspects a better immune response.
“So it will be interesting to see if we see that in humans also.”
The Com-COV study was expanded in April to also look at mixing Moderna and Novavax vaccines.
Prof Snape said: “The main drive behind this study is to increase the flexibility of the UK’s schedule and resilience in the case of problems with supply or availability of any of the vaccines.”









