THE FIRST Covid vaccine was given to a bunch of Britons this morning, with millions more to follow by the end of this year.
And the Prime Minister has reminded people to not be “scared” of taking the jab from the drug giant Pfizer.
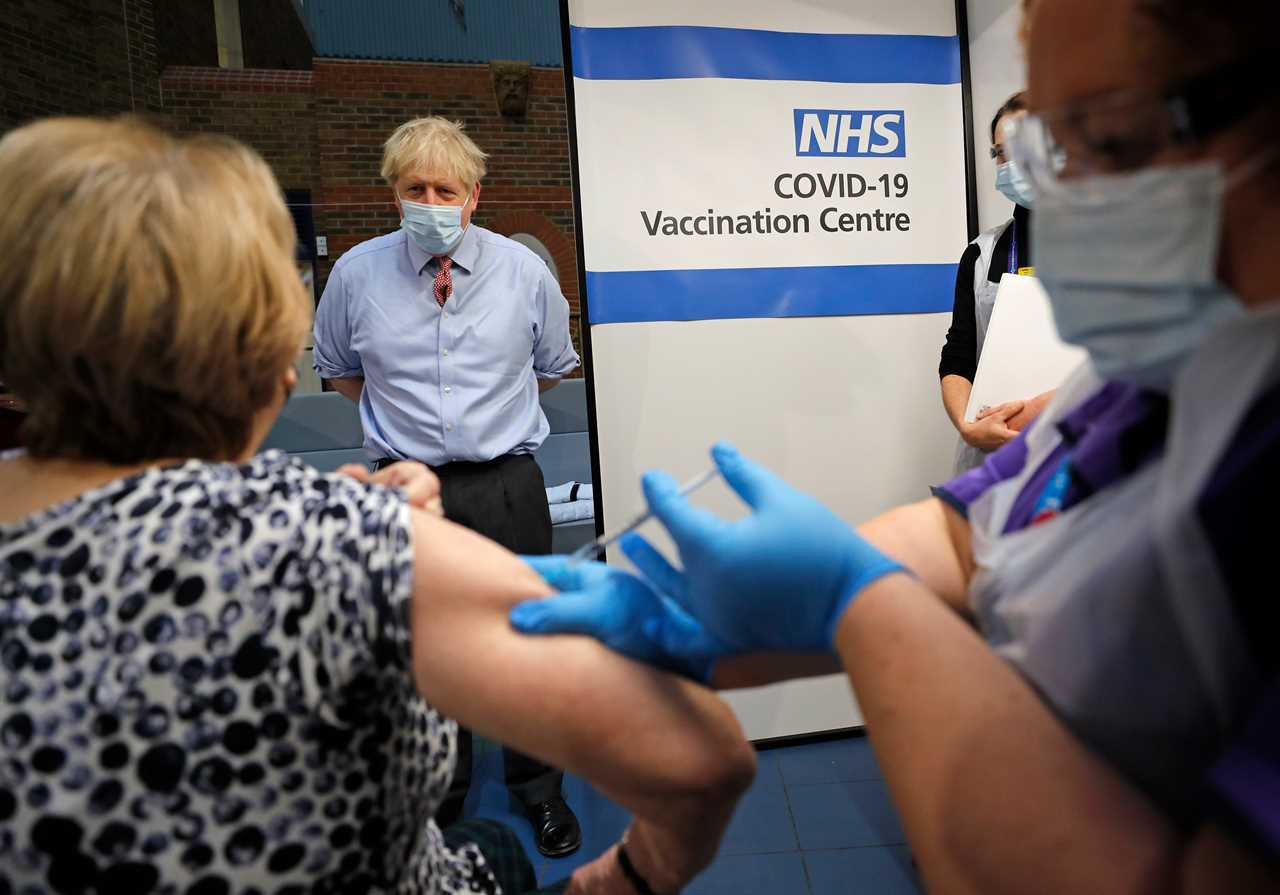
Boris Johnson said people objecting to having the jab are “totally wrong” to do so, amid fears anti-vaxxing campaigns will deter people from accepting an offer to receive the vaccine.
Speaking at the vaccination centre at Guy’s Hospital in London on Tuesday, the PM said it was moving to talk to Lyn Wheeler, 81, who was the first to receive the vaccine there.
The PM said: “To all those who are scared (of getting vaccinated) – don’t be. You have seen Lyn (Wheeler) take it, you have seen people take the vaccine this morning in large numbers.
“There’s nothing to be nervous about.”
He added: “What I would say is that there are those obviously who feel that a vaccine is something they object to politically or for ideological reasons.
“I think they are totally wrong. It’s safe, it’s the right thing to do, it’s good for you and it’s good for the whole country.”
Lyn, from Bromley, said it had been “lovely” to take part in the Covid-19 vaccination programme, and that she hoped to inspire others to do the same.
She said: “We have got to do something, we can’t go on as we are. We can’t continually go around being afraid to go to the shops or being afraid to sit on a bus.
“You have got to realise that life is a bit if a risk and you can’t keep hiding away, you have to stand up and go for things.
“I’m going for it because I feel there’s no other way forward, we can’t keep sitting in our houses.”

Prof Stephen Powis, NHS England’s national medical director, has also reassured the vaccine is safe.
Asked what his message was to people who might have concerns over the vaccine on BBC Breakfast, he said: “Vaccination is one of the safest forms of medicine.
“I’m absolutely confident that this … all vaccines are safe. And so if you get called, we’ll be calling you to come and get it, then my advice is come and get it.
“We know they work. This one has been tested in many thousands of people in clinical trials.
“And, of course, the independent regulator, the MHRA, has looked at it carefully, as it always does, and has given it the green light.”
The Pfizer jab, made with German biotechnology company BioNTech, was approved by the Medicines and Healthcare products Regulatory Agency (MHRA) last week.
Officials announced the jab was safe and effective on December 2, a moment described as “historic”.
It means the UK is the first country in the world to have an approved vaccine, less than a year after Covid-19 emerged.
Speaking on the day it was approved, Dr June Raine, of the MHRA medicines regulator, stressed the vaccine met “rigorous high standards”.
“The public’s safety has always been at the forefront of our minds,” she said.
How do they know if it safe?
Safety trials begin in the lab, with tests on cells and animals first, before moving on to human studies.
The human trials for the Pfizer/BioNtech jab involved some 43,000 volunteers – that’s a lot of people.
Professor Jonathan Van Tam, deputy chief medical officer, told The Mirror: “These are very, very big studies.
“The numbers involved were essentially the same as you’d expect for a normal peacetime vaccine, and on top of that the safety assessments and the assessments of effectiveness at the end are the same.”
Approval will only be given for a vaccine in the UK if the government regulator, the Medicines & Healthcare products Regulatory Agency (MHRA), deems it safe and effective to do so.
When the jab was given its MHRA stamp of approval, Dr Raine said more than 1,000 pages of data had been examined, and experts had worked “round the clock, carefully, methodically poring over tables and analyses and graphs on every single piece of data”.
She added: “This recommendation has only been given by the MHRA following the most rigorous scientific assessment of every piece of data so that it meets the required strict standards of safety, of effectiveness and of quality.”
95 per cent effective
Tests found it was 95 per cent effective at preventing Covid-19, and scientists found no serious side effects.
In the Pfizer trials around 20,000 people were given the vaccine, and 20,000 were given a dummy jab.
Of those vaccinated, just eight caught the coronavirus compared with 164 people who fell ill when taking the placebo drug.
Nine became severely ill from the coronavirus, and they had all been given the placebo.

The findings mean that of 100 people given the vaccine, 95 will be protected from Covid-19 – an efficacy of 95 per cent.
It’s not known why some people didn’t respond positively to the vaccine.
However, a success rate of 95 per cent is similar to other jabs and is much higher than scientists expected from a coronavirus vaccine, considering no vaccine has ever successfully been made against other human coronaviruses.
What’s unknown at this stage is if the vaccine stops people from passing the virus on to others, even if it does not making them sick. It’s very difficult to study this in a trial.
Sir Patrick Vallance, the chief scientific advisor for the UK, said today this will become clearer over the next few months.
Dr Raine said members of the public would be invited to take part in a monitoring process post-vaccine to help them build a “body of knowledge” on the vaccine.
There are some indications that the Oxford/AstraZeneca jab may prevent transmission because the study regulary tested participants for the virus.
Prof Van Tam told The Mirror: “If you’re going to prevent transmission you need a vaccine that is taking out the illness and also the asymptomatic infections. There are some signs from AstraZeneca that this might be the case.
“That does not mean the other vaccines where results have already been announced or that are or are still in development, don’t also reduce transmission; it’s just we don’t know at this point in time.
“We hope they will because they’ll have a bigger effect if they can take out transmission.”
What are the side effects?
The evidence shows any side effects are “mild” and “rare”, and are very similar to those seen with the flu jab.
Scientists at Pfizer said their extensive trials showed the vaccine is “generally well tolerated in all age groups”.
Side effects can be mild and independent data monitoring reported that there were no safety concerns.
Speaking to Trending In The News, Dr Sarah Jarvis, GP and Clinical Director of Patientaccess.com said all vaccines cause side effects in some people – but that this is largely because they are designed to boost your immune system.
Dr Sarah said: “When your immune system is learning to fight off an invader, lots of white blood cells rush to where they’re needed and produce natural chemicals.
“That means most vaccines, including the annual flu vaccine, can lead to mild redness, tenderness of swelling around the area you had the injection.
“In the Covid-19 vaccine trials, all the side effects seen so far have been mild and very similar to those seen with other vaccines like the yearly flu vaccine.”
The vaccine is administered to a patient in two doses and reports from Pfizer state that the worst side effects were fatigue and headaches – but only after the second dose.
Just four per cent of people reported fatigue and two per cent reported a headache.
Similar to jabs such as the flu, some people reported pain in the site the injection was administered.
Dr Sarah added that if you do get these symptoms then they will “settle down within a few days”.
Chief medical officer at BioNTech, Özlem Türeci said that these are “common reactions you would have with any vaccination”.
While there were few side effects in those who trialled the jab, there were fewer and milder side effects in older adults.
But wasn’t the jab rushed?
Some people have concerns the vaccine was made too quickly and corners were cut when checking the data, and therefore cannot be safe.
It didn’t help that America’s top infectious disease expert Dr Anthony Fauci, the director of the US National Institute of Allergy and Infectious Diseases, said on December 4: “They [Britain] really rushed through that approval.”
The MHRA defended itself against criticism, saying it has “rigorously assessed the data” for the Pfizer/BioNtech Covid-19 vaccine.
In a later interview with BBC News, Dr Fauci said he did not mean to “imply any sloppiness”, adding: “I do have great faith in both the scientific community and the regulatory community at the UK.”







