PEOPLE with “significant allergies” have been warned by officials not to take the Covid-19 vaccine.
Two NHS workers who received the landmark Pfizer Covid jab on V Day have suffered allergic reactions needing treatment.
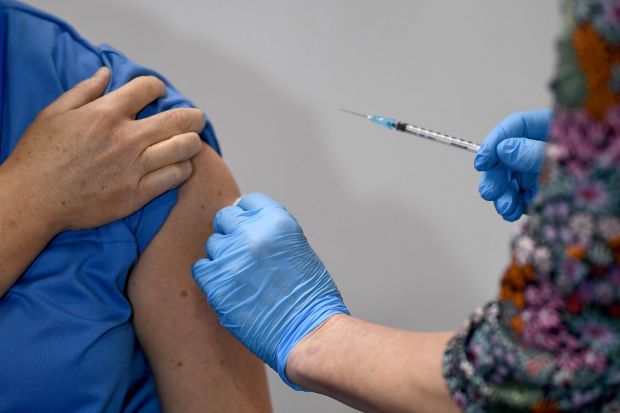
The staff members both had a “significant history of allergic reactions” to the extent where they need to carry an EpiPen or similar device, the NHS confirmed.
They developed symptoms of “anaphylactoid reaction” shortly after receiving the vaccine.
NHS England confirmed both are now recovering and all trusts involved with the vaccination programme have been informed.
Regulators at the MHRA have now warned anyone else with a history of “significant” allergic reactions to medicines, food or vaccines should not receive the jab.
“Significant” means a person has suffered anaphylaxis – a potentially life-threatening reaction which can cause breathing difficulties, confusion, vomiting or collapse – or needs to carry a an adrenaline autoinjector.
Anyone scheduled to receive the vaccine on Wednesday will be asked about their history of allergic reactions.
The MHRA advice states: “Any person with a history of a significant allergic reaction to a vaccine, medicine or food (such as previous history of anaphylactoid reaction or those who have been advised to carry an adrenaline autoinjector) should not receive the Pfizer/BioNtech vaccine.
“Resuscitation facilities should be available at all times for all vaccinations. Vaccination should only be carried out in facilities where resuscitation measures are available.”
The news will come as a huge blow to people with allergies.
An estimated 10 to 40 per cent of the population have an allergy, according to Allergy UK. However most of these will not be severe.
Over 200,000 people in the UK, including children, have severe allergies that require a prescribed emergency adrenaline, according to the National Institute of Health and Clinical Excellence.
Some of the most common allergies, according to the NHS, are to foods like nuts, fruit, shellfish, eggs and cow’s milk, and medicines such as ibuprofen, antibiotics and penicillin.
Professor Stephen Powis, national medical director for the NHS in England, said: “As is common with new vaccines the MHRA have advised on a precautionary basis that people with a significant history of allergic reactions do not receive this vaccination after two people with a history of significant allergic reactions responded adversely yesterday.
“Both are recovering well.”
The two NHS staff members were among thousands, of Brits who became the first people in the world to receive the Pfizer vaccine yesterday.
Elderly British patients who were first in line to get the jab urged sceptics to take the vaccine for the good of the country.

The MHRA added should anyone else given the jab suffer any suspected adverse reactions, to report it via the Yellow Card scheme.
Dr June Raine, chief executive of the MHRA, said today the cases were being investigated after regulators were told of the reactions on Tuesday night.
She added: “I may share with the committees that even last evening, we were looking at two case reports of allergic reactions.
“We know from the very extensive clinical trials that this wasn’t a feature.”
Pfizer said today the vaccine was “well tolerated” during the trials with “no serious safety concerns”.
A spokeswoman for Pfizer said: “We have been advised by MHRA of two yellow card reports that may be associated with allergic reaction due to administration of the Covid-19 BNT162b2 vaccine.
“As a precautionary measure, the MHRA has issued temporary guidance to the NHS while it conducts an investigation in order to fully understand each case and its causes. Pfizer and BioNTech are supporting the MHRA in the investigation.”
Pfizer said the trial has enrolled over 44,000 participants to date, over 42,000 of whom have received a second vaccination.
It is not clear if the trials of the Pfizer/BioNTech jab included volunteers with known allergies. The final results are not published in a medical journal.
Paul Hunter, a professor in medicine at University of East Anglia, told the Sun: “The Oxford vaccine has now published an analysis of its Phase 3 trials and given more detailed reporting of the side effects. But Pfizer have yet to do so. So I do not know whether or not they included or excluded people with a history of severe allergy.
“Allergic reactions to vaccines do occur and are immunizations are often contraindicated in for people who have had a previous severe reaction.
“This is why people who have had the vaccine are required to hang around for a while after having had their injection. If you are still in the clinic an allergic reaction is much easier to deal with.
“I don’t think this is particularly unexpected and I do not think it should interfere with roll out of the vaccination programme.”
Dr Andrew Preston, a reader in microbial pathogenesis, Department of Biology and Biochemistry at University of Bath, said: “Allergic reactions arise from a trigger of an aspect of the immune system by a specific stimulus.
“So it will be a case of working out what the signal is from within the vaccine.
“Odd that none were detected during the Phase 3 trials.”
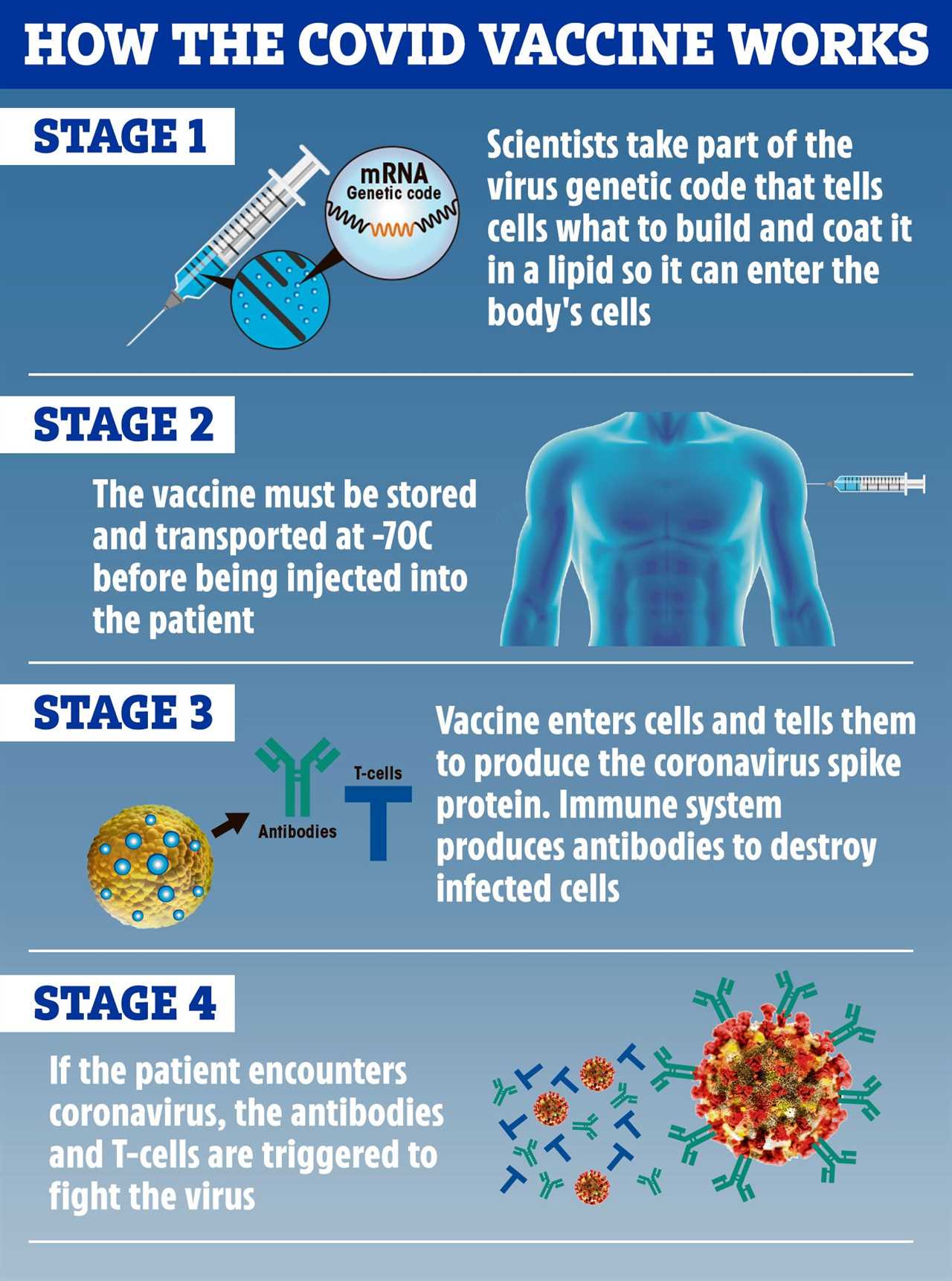
The vaccine uses mRNA technology which has never been approved for use before. However, it has been around for years and tested in humans for at least four infectious diseases: rabies, influenza, cytomegalovirus, and Zika.