BRAZILIAN President Jair Bolsonaro said he will refuse a coronavirus jab even after it gets the green light from his own government.
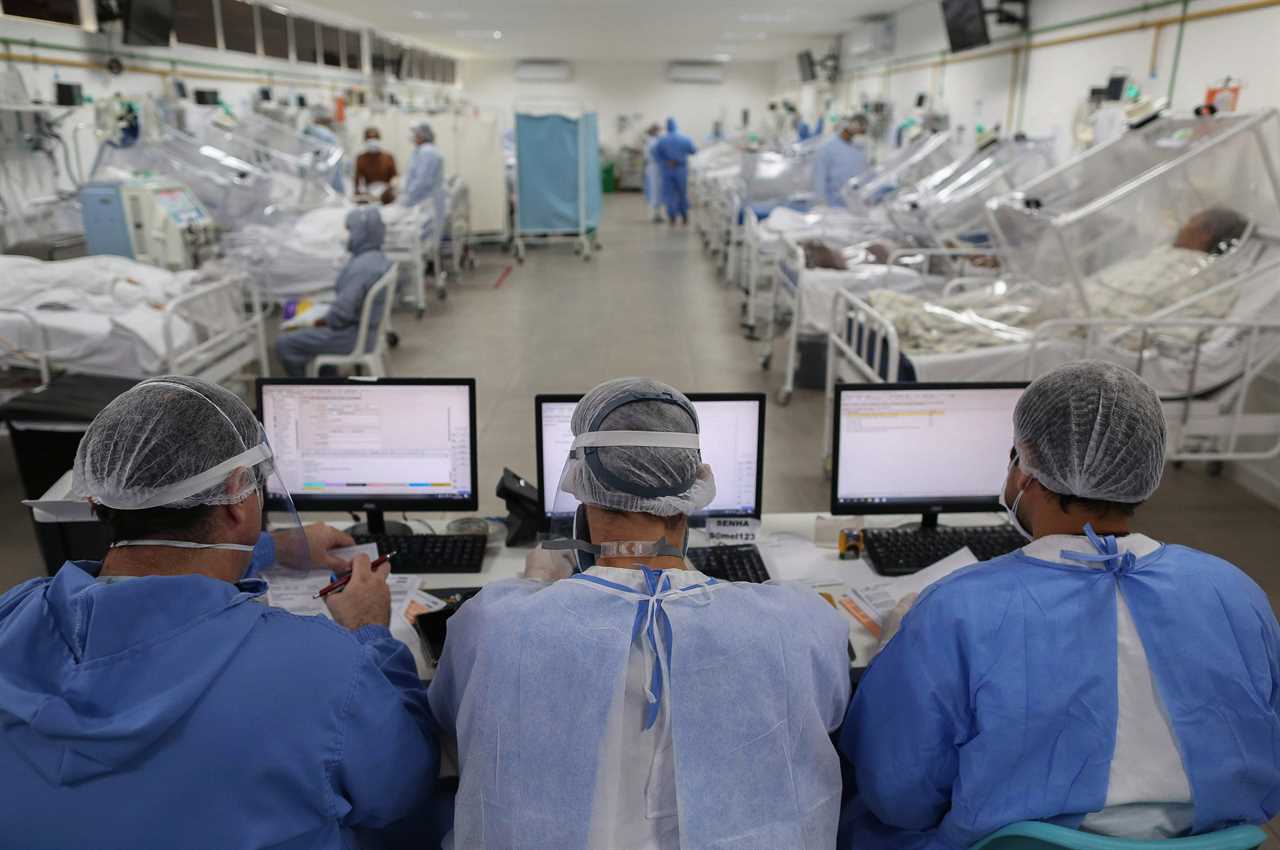
Brazil has recorded 170,000 coronavirus deaths – the second highest death toll in the world – but Bolsonaro said he is “sure” that vaccination against the deadly virus will not be made compulsory in the country.

“I’m telling you, I won’t take it (the vaccine),” he said in a video posted to social media on Thursday. “It is my right.”
Bolsonaro contracted Covid-19 at the beginning of July, and more than half of his cabinet have tested positive for the virus in recent months.
He has faced criticism for his handling of the pandemic, including playing down the virus, opposing lockdown measures and promoting hydroxychloroquine despite studies showing it is ineffective against coronavirus.
Earlier this year, Bolsonaro urged state governors to reopen their economies despite health officials’ recommendations, calling the pandemic “little flu”.
He has famously been flouting all social distancing measures as he believes that the economic backlash is more important than the public health crisis.
Once any treatment is approved by Brazil’s health regulatory authorities, his government will “immediately organise” its purchase and distribution to those who want it, he said.
But he said he was “sure” that Brazil’s parliament would not make vaccination mandatory.
VACCINE RACE
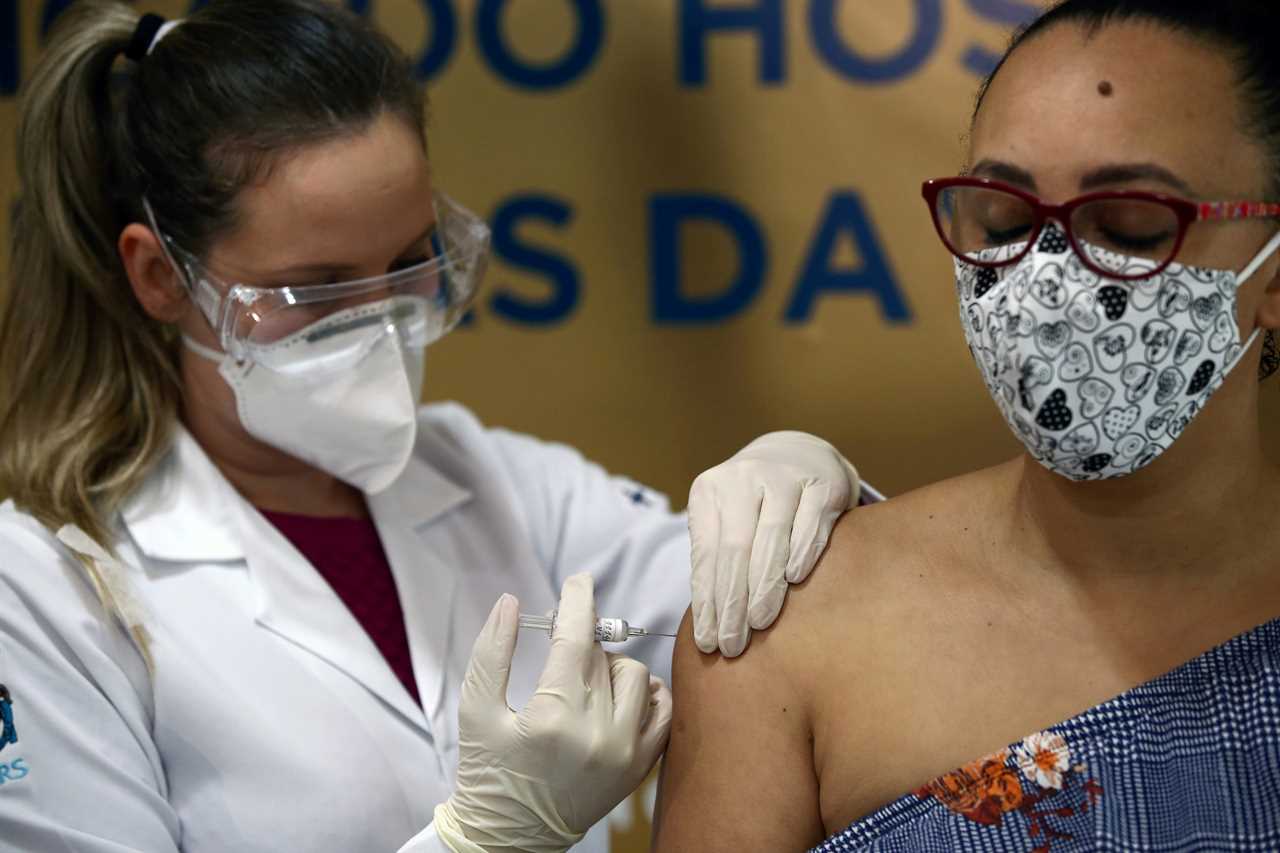
Many nations are pinning their hopes on a vaccine to end the pandemic, with two jabs on the cusp of approval by authorities after showing about 95 percent efficacy in trials.
Brazil’s health ministry has already agreed to buy 100 million doses of the vaccine developed by AstraZeneca and Oxford University.
More than 60.9 million people have been infected by Covid-19 around the world, and 1.4 million have died, according to an AFP tally on Friday.
More than 10,859 people have died in the last 24 hours, with 561,041 new cases reported worldwide.
The United States is the worst-affected country with 263,462 deaths, followed by Brazil with 171,460 deaths, India with 135,715, Mexico with 104,242, and the United Kingdom with 57,031.
In the global race to develop vaccines against coronavirus, AstraZeneca’s candidate is viewed as offering one of the best hopes for many developing countries because of its cheaper price and ability to be transported at normal fridge temperatures.



Elsewhere, officials in the Philippines said they would secure 2.6 million shots of the AstraZeneca shot – the country’s first supply deal for a Covid-19 vaccine.
The Thai government also signed a deal on Friday to procure 26 million doses of the vaccine to fight the pandemic.
The announcements came despite some scientists raising doubts about the robustness of results showing the shot was 90 per cent effective in a sub-group of trial participants who, by error initially, received a half dose followed by a full dose.
AstraZeneca released trial data on Monday which showed its experimental vaccine prevented on average 70 per cent of COVID-19 cases in late-stage trials in Britain and Brazil.
While the success rate was 90 per cent in the sub-group, some experts said the relatively small number of participants made it harder to be confident in the findings.
Only 2,741 volunteers were in the sub-group of the AstraZeneca-Oxford trial that gave the 90 per cent efficacy result – a fraction of the tens of thousands in trials for Pfizer-BioNTech’s and Moderna’s vaccines.
AstraZeneca said the administering of the half dose in the trial had been reviewed and approved by independent data safety monitors and the UK regulator, adding that the regulator publicly confirmed there was “no concern”.
The European Medicines Agency said on Thursday it would “assess data on the efficacy and safety of the vaccine in the coming weeks once they have been received from the company”.
The UK government, which has secured 100 million doses of the vaccine, has targeted a rollout to begin before Christmas.
“We have formally asked the regulator to assess the Oxford/AstraZeneca vaccine, to understand the data and determine whether it meets rigorous safety standards,” Health Secretary Matt Hancock said.
“This letter is an important step towards deploying a vaccine as quickly as safely possible.”








