THE University of Oxford has paused trials of its AstraZeneca vaccine on children and teenagers over concerns about very rare blood clots.
The tests on youngsters aged six to 17 years old were halted while UK regulators investigate possible links to blood clots in young adults.

Read our coronavirus live blog for the latest news & updates…

A senior government jabs adviser has suggested the vaccine rollout should be paused for younger people until regulators have issued firm guidance on the Oxford/AstraZeneca jab’s safety.
The University of Oxford trials began in February with around 300 volunteers signing up.
The recruiting of new participants has been halted, but Oxford said there was “no safety concerns” during the trials and the pause was precautionary.
Boris Johnson today defended the AstraZeneca vaccine after concerns were raised in Europe about its rollout as the PM urged Brits to get jabbed.
‘NO SAFETY CONCERNS’
Regulators in the UK and the European Union have both stated the AstraZeneca vaccine is “safe and effective”, while they carry out ongoing reviews.
The UK’s regulatory body, the MHRA (The Medicines and Healthcare products Regulatory Agency), is said to be meeting today to discuss issues surrounding blood clots and what age groups the jab should be given to.
After the trials were paused, a University of Oxford spokesman said: “Whilst there are no safety concerns in the paediatric clinical trial, we await additional information from the MHRA on its review of rare cases of thrombosis/thrombocytopaenia that have been reported in adults, before giving any further vaccinations in the trial.
“Parents and children should continue to attend all scheduled visits and can contact the trial sites if they have any questions.”
The trial was set up so scientists can examine whether the vaccine has a similar immune response in kids.
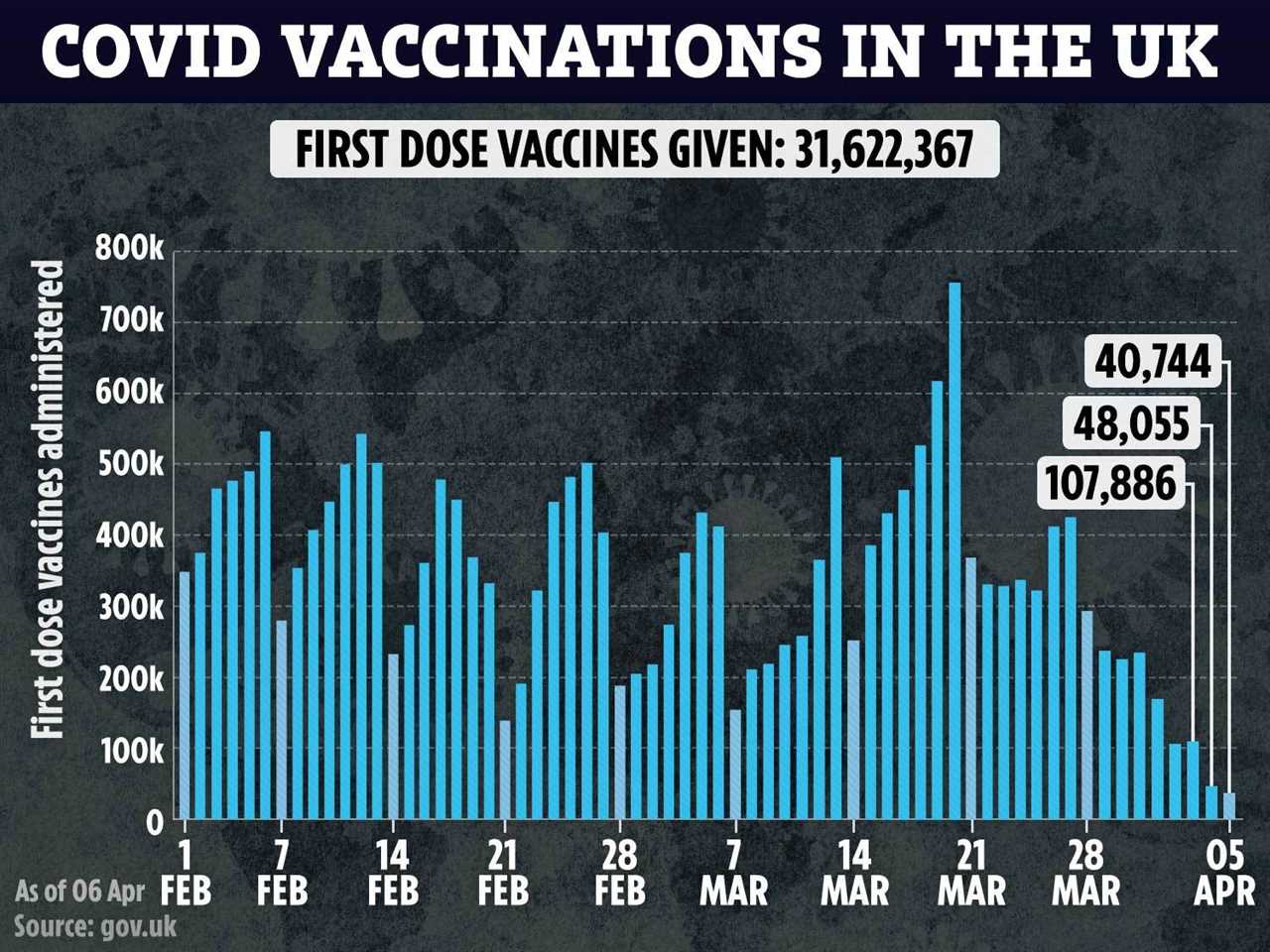
The UK’s bumper vaccine rollout has seen more than 31.6 million Brits have a first jab, with 5.4 million people also having a second dose.
As the MHRA probes if there is a link between the Oxford/AstraZeneca jab and rare blood clots, a government expert suggested that phase two of the government’s vaccine rollout – which will see adults under-50 given their doses – should be slowed down.
Dr Maggie Wearmouth, who sits on the joint committee on vaccination and immunisation (JCVI), said: “We have to show that perhaps slowing things down, not rolling out phase two at this stage… until we’re absolutely certain.
“The issue is about safety and public confidence. We don’t want to cover anything up that we feel that the public should be knowing.
“We’re not here to blindly follow targets or due dates. We will do what is necessary for the British public.”
Sage adviser Professor Calum Semple urged people to continue getting the Oxford/AstraZeneca jabs and said the risk of a clot in adults is “probably one in a million”.
He told Channel 4 News: “This has been done out of exceptional caution and the big story still is that for a middle-aged, slightly overweight man, such as myself, my risk of death is one in 13,000 – the risk of this rare clot, which might not even be associated with the vaccine, is probably one in a million.
“So I’m still going to say it’s better to get the vaccine than not get the vaccine and we can pause and take time to carefully consider the value for children because they’re not at risk of death from Covid.”
He added: “If you’ve been called for the vaccine then you’re in an age group that is very likely to benefit from the vaccine.
“So the bottom line is if you’ve been called for the vaccine I would urge you to take the vaccine.”
Earlier, a European Medicines Agency (EMA) official claimed there is a “link” between the AstraZeneca Covid vaccine and rare blood clots.
Speaking to Italy’s Il Messaggero newspaper, the EMA’s head of vaccines Marco Cavaleri said it is not yet clear what is causing blood clots from the jab.
Mr Cavaleri said: “In my opinion, we can say it now, it is clear there is a link with the vaccine. But we still do not know what causes this reaction.
“In the next few hours, we will say that there is a connection, but we still have to understand how this happens.”
The EMA’s safety committee said it had “not yet reached a conclusion and the review is currently ongoing”.
The Prime Minister toured AstraZeneca’s Macclesfield plant as ministers reassured people to continue taking the shot while the medicines watchdog probes “very rare” cases of blood clots in some younger recipients.
Mr Johnson told reporters at it was “very important to stress that the best thing of all is to vaccinate our population, get everybody out getting the jab.
“That’s the key thing and that’s what I would advocate, number one”.


Did you miss our previous article...
https://trendinginthenews.com/covid-19/when-can-mobile-hairdressers-work-from






