AN ARTHRITIS drug that reduces the risk of a patient dying of the coronavirus by 24 per cent will now be given on the NHS, the Government has announced.
Patients in intensive care units across the UK will receive tocilizumab and sarilumab from as soon as tomorrow, after official guidance is sent out to NHS Trusts across the country.
Data from the government’s REMAP-CAP clinical trial found that the drugs can reduce the time spent in hospital by up to ten days and in turn, could lift some of the pressure currently placed on the health service.
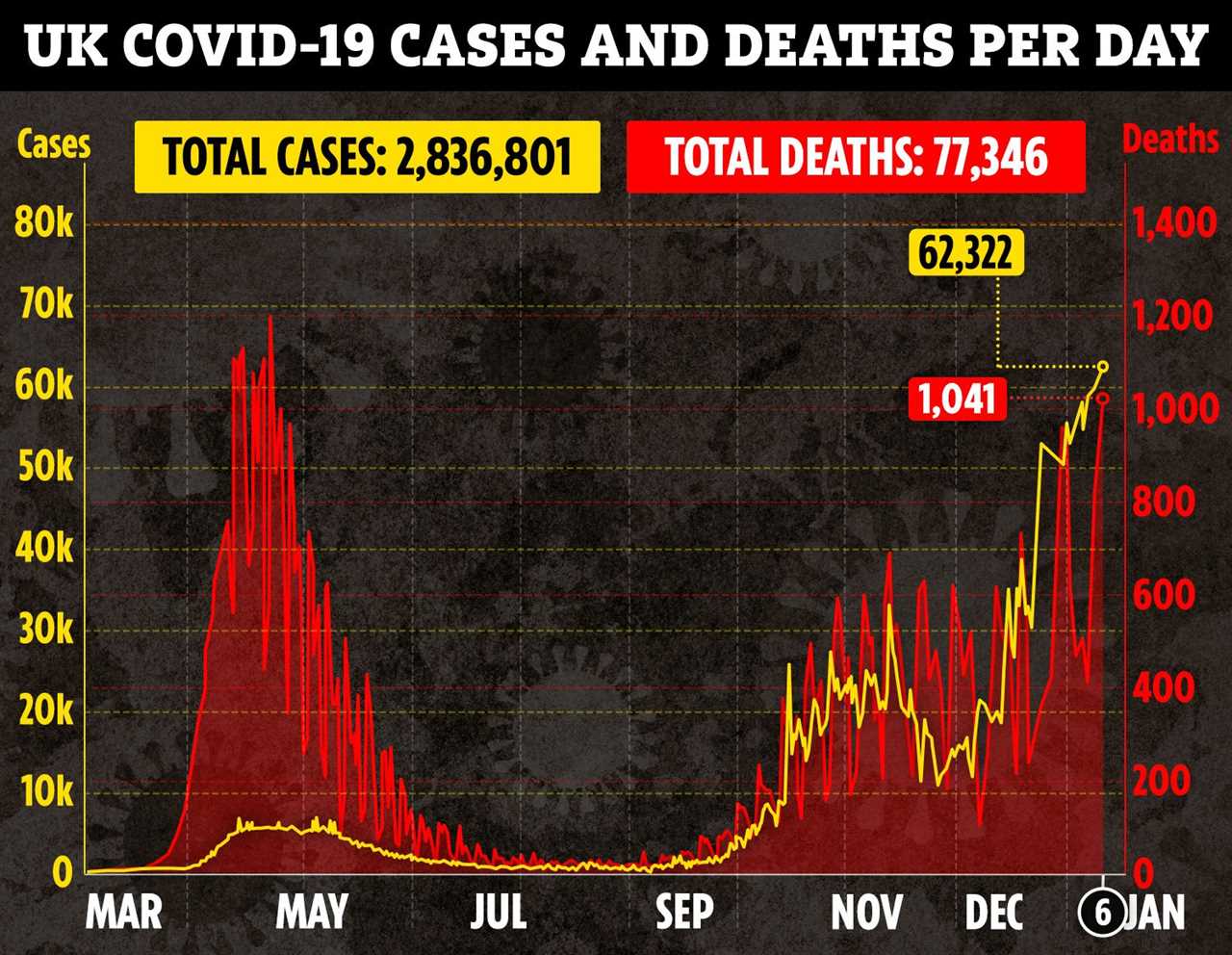
As of January 4, official data states that there are 30,451 people in hospital with the coronavirus across the UK.
The research, published today shows that treatment can reduce the risk of death by 24 per cent when given to patients within 24 hours of them entering intensive care.
Professor Jonathan Van Tam said the use of the drug was a “significant step forward for increasing survival of patients in intensive care with Covid-19”.
He added: “The data shows that tocilizumab, and likely sarilumab, speed up and improve the odds of recovery in intensive care, which is crucial for helping to relieve pressure on intensive care and hospitals and saving lives. “
However, the government stated that most of the data had been sourced when drugs were given alongside other treatments such as dexamethasone.
Dexamethasone was approved for use on Covid patients in June last year.
The trial, led by Imperial College London and the Intensive Care National Audit & Research Centre found that using just dexamethazone alone would mean the rate of death was at 35 per cent.
Using tocilizumab alongside it meant this dropped to 28 per cent, meaning patients were less likely to die when both drugs were used together.
The treatment is usually given to patients who have rheumatoid arthritis and these patients were found to have left intensive care between seven to 10 days earlier than those who were not given the treatment.
The drugs are already being used across the NHS and official guidance will be issued tomorrow – urging clinicians to use tocilizumab for Covid patients who are admitted to intensive care.
At the time of full analysis, 353 patients had been assigned to tocilizumab, and 48 to sarilumab.
The majority of patients were also treated with corticosteroids and were receiving respiratory support.

GOOD NEWS
Patients involved in the trial included those who were “less severely ill” with Covid-19 and some patients started the treatment at different stages of the illness.
One expert however, questioned the treatment and how it would work on different types of patients.
Prof Martin Landray, Professor of Medicine & Epidemiology, Nuffield Department of Population Health, University of Oxford said while the result of the trial was “good news” there were still questions that needed to be answered.
He said: “In contrast to previous trials, REMAP-CAP also reports lower mortality among patients given tocilizumab.
“There are, of course, still unanswered questions. For example, exactly how well does tocilizumab work in different types of patients?
“And if given earlier, might it reduce the need for patients to require mechanical ventilation in the first place?
“We don’t yet know the answers. But, REMAP-CAP has injected that bit of optimism we all need.”
Professor Anthony Gordon, Chair in Anaesthesia and Critical Care at Imperial College London and a Consultant in Intensive Care Medicine at Imperial College Healthcare NHS Trust said the findings would have “significant implications” for Covid patients.
He added: “We found that among critically ill adult patients – those receiving breathing support in intensive care – treatment with these drugs can improve their chances of survival and recovery.
“At a time when hospitalisations and deaths from Covid-19 are soaring in the UK, it’s crucial we continue to identify effective treatments which can help to turn the tide against this disease.”
The Department of Health and Social Care said it was working closely with the supplier, Roche, to ensure that the drugs continue to be available to hospitals across the UK.
Health and Social Care Secretary Matt Hancock today said that the UK have proven “time and time again” that it is is at the forefront of “innovative treatments”.
He added: “Today’s results are yet another landmark development in finding a way out of this pandemic and, when added to the armoury of vaccines and treatments already being rolled out, will play a significant role in defeating this virus.
“We have worked quickly to ensure this treatment is available to NHS patients without delay, meaning hundreds of lives will be saved.”
The government has so far dedicated £1.2 million to the REMAP-CAP trial but this latest study has not yet been peer reviewed.
Tocilizumab will be used to reduce mortality rates in Covid patients and will be administered intravenously in a one or two dose regime.
While the drugs will now be used for Covid patients and will help relieve some of the huge challenges the NHS is facing, experts have urged people to continue to follow the rules set out by the government.
Professor Stephen Powis, NHS national medical director said: “The fact there is now another drug that can help to reduce mortality for patients with Covid-19 is hugely welcome news and another positive development in the continued fight against the virus.
“This signals how the NHS is working all the time to find new treatments and therapies, but the best advice for individuals is to remember the hands, face, space guidance.”
So far over 3,900 patients in 15 countries have been enrolled at more than 290 hospitals worldwide to the trial which has helped shape of health professionals deliver treatments for the coronavirus.
Did you miss our previous article...
https://trendinginthenews.com/covid-19/who-is-brigadier-phil-prosser






