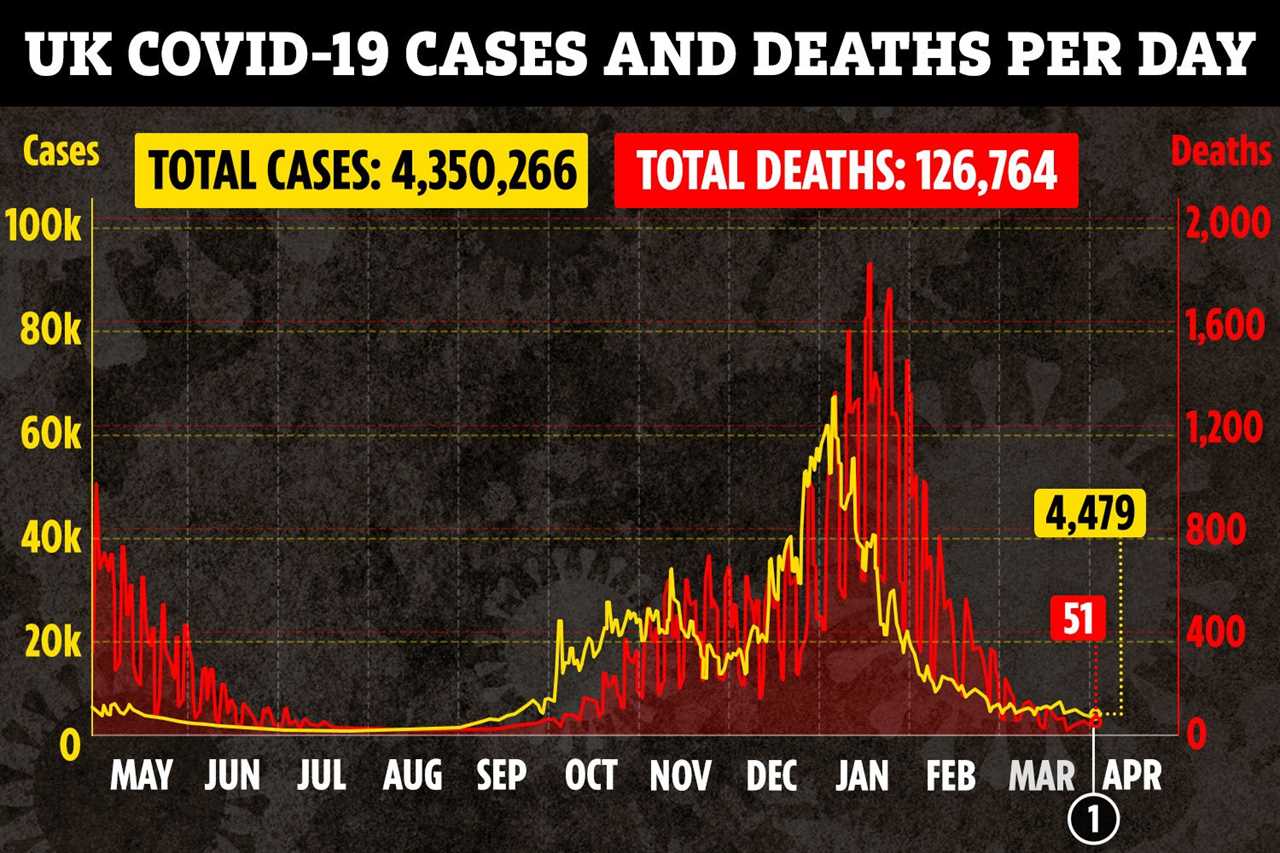
A HEALTH regulator has now received a total of 30 reports of rare blood clots from people who have had the Oxford/AstraZeneca jab in the UK.
None of those given the Pfizer vaccine reported a clot, the Medicines and Healthcare products Regulatory Agency (MHRA) revealed.

Read our coronavirus live blog for the latest news & updates…

News of patients suffering severe and very rare clotting after receiving the jab was reported as countries around Europe hold back on recommending the dose for younger age groups.
Initially, on March 18, the UK regulator said there had been five cases of the brain clot among 11m administered shots.
But tonight, it said 25 more clotting cases have been reported – 22 of which were the rare ailment.
It comes as:
- Brits have been warned we could all face a fourth lockdown if huge groups meet over Easter
- Millions of Brits told book your Covid vaccine now without delay, by NHS chief
- Brits will likely have to take Covid tests or show immunity to go to the pub, says Boris
- EU chief warns Britain will get ‘ZERO’ AstraZeneca jabs until supplies are met saying ‘there’s nothing to negotiate’
- Easter Covid lockdown rules: What you can and can’t do over the UK bank holiday
Just a fortnight ago, the European Medicines Agency (EMA) concluded the AstraZeneca vaccine was safe.
But on Wednesday, Chancellor Angela Merkel announced that while she’ll take the British vaccine, it’s being partially suspended for younger people.
In a statement released before her announcement, AstraZeneca said it is analysing its own records to understand whether “the rare blood clots reported occur more commonly than would be expected naturally in a population of millions of people”.
People across the world have had the AstraZeneca vaccine without any complications.
Regulators have stressed that the benefits of the jab far outweigh any potential risks.

The condition, called CVST, occurred with low blood platelets and is an extremely rare combination of events.
It’s so rare, UK regulators at the MHRA said they did not know how often it happens in the general population. While investigations continue, people have been urged to accept their vaccine offer when it comes.
Similar reports of rare blood clots have caused France, Sweden, Finland and Canada, as well as Germany, to recommend that younger people, who are much more likely to be affected by the condition, avoid the shot.
The jab is suspended altogether in Norway, where of the 120,000 recipients, six suffered clots and four died. It’s also not in use in Denmark.
In Germany, 31 cases have been reported after 2.7m vaccinations, including 29 women aged between 20 and 63, and two men aged 36 and 57.






